
Ēā»ÆŃĒĶ(CuH)ŹĒŅ»ÖÖÄŃČÜĪļÖŹ,ÓĆCuSO4ČÜŅŗŗĶ”°ĮķŅ»ĪļÖŹ”±ŌŚ40”«50 ”ꏱ·“Ó¦æÉÉś³ÉĖü”£CuH²»ĪȶØ,Ņ×·Ö½ā;ŌŚĀČĘųÖŠÄÜČ¼ÉÕ;ÓėĻ”ŃĪĖį·“Ó¦ÄÜÉś³ÉĘųĢå;Cu+ŌŚĖįŠŌĢõ¼žĻĀ·¢ÉśµÄ·“Ó¦ŹĒ2Cu+ Cu2++Cu”£øł¾ŻŅŌÉĻŠÅĻ¢,½įŗĻ×Ō¼ŗĖłÕĘĪյĻÆѧÖŖŹ¶,»Ų“š:
Cu2++Cu”£øł¾ŻŅŌÉĻŠÅĻ¢,½įŗĻ×Ō¼ŗĖłÕĘĪյĻÆѧÖŖŹ¶,»Ų“š:
(1)ÓĆCuSO4ČÜŅŗŗĶ”°ĮķŅ»ĪļÖŹ”±ÖĘCuHµÄ·“Ó¦ÖŠ,ÓĆŃõ»Æ»¹Ō¹Ūµć·ÖĪö,Õā”°ĮķŅ»ĪļÖŹ”±ŌŚ·“Ó¦ÖŠĖłĘšµÄ×÷ÓĆŹĒ”””””””””””””””””””””””£
(2)Š“³öCuHŌŚĀČĘųÖŠČ¼ÉյĻÆѧ·“Ó¦·½³ĢŹ½
(3)CuHČܽāŌŚĻ”ŃĪĖįÖŠÉś³ÉµÄĘųĢåŹĒ””””””””,Čē¹ū·“Ó¦ÖŠÉś³ÉĮĖ±ź×¼×“æöĻĀ22.4 LµÄĘųĢå,±»»¹ŌµÄĄė×ӵƵē×ÓµÄĪļÖŹµÄĮæŹĒ”””””””””£
 ĆæČÕ10·ÖÖÓæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
ĆæČÕ10·ÖÖÓæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻņFeI2ČÜŅŗÖŠ²»¶ĻĶØČėCl2£¬ČÜŅŗÖŠI£”¢I2”¢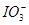 ”¢Fe2£«”¢Fe3£«µČĮ£×ÓµÄĪļÖŹµÄĮæĖęn(Cl2)”Ćn(FeI2)±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾”£
”¢Fe2£«”¢Fe3£«µČĮ£×ÓµÄĪļÖŹµÄĮæĖęn(Cl2)”Ćn(FeI2)±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾”£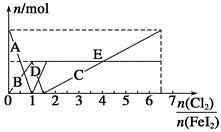
ŅŃÖŖ£ŗ2Fe3£«£«2I£=I2£«2Fe2£«”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Öø³öĶ¼ÖŠÕŪĻßEŗĶĻ߶ĪCĖł±ķŹ¾µÄŅāŅå£ŗÕŪĻßE±ķŹ¾ £»Ļ߶ĪC±ķŹ¾ ”£
(2)Š“³öĻ߶ĪDĖł±ķŹ¾µÄ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
(3)µ±n(Cl2)”Ćn(FeI2)£½6£®5Ź±£¬ČÜŅŗÖŠn(Cl£)”Ćn(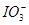 )£½ ”£
)£½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
äåµÄŃõ»ÆŠŌ½éÓŚĀČŗĶµāÖ®¼ä£¬ĄūÓĆÕāŅ»ŠŌÖŹ½ā¾öĻĀĆęµÄĪŹĢā”£
(1)ÄćČĻĪŖ£ŗ½«ŗ¬ÓŠĻĀĮŠÄÄÖÖ·Ö×Ó»ņĄė×ӵďŌ¼Į¼ÓČėµ½ŗ¬ÓŠBr£µÄČÜŅŗÖŠ£¬æÉŅŌ½«Br£Ńõ»ÆĪŖBr2__________”£
| A£®I2 | B£®I | C£®Cl2 | D£®Cl£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©½«·Ļ·°“߻ƼĮ(Ö÷ŅŖ³É·ÖV2O5)ÓėĻ”ĮņĖį”¢ŃĒĮņĖį¼ŲČÜŅŗ»ģŗĻ£¬³ä·Ö·“Ó¦£¬ĖłµĆČÜŅŗĻŌĖįŠŌ£¬ŗ¬VO2£«”¢K£«”¢SO42-µČ”£Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½_________________________________”£
£Ø2£©ĻņÉĻŹöĖłµĆČÜŅŗÖŠ¼ÓČėKClO3ČÜŅŗ£¬³ä·Ö·“Ó¦ŗó£¬ČÜŅŗÖŠŠĀŌö¼ÓĮĖVO2+”¢Cl£”£Š“³ö²¢ÅäĘ½øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½£¬²¢±ź³öµē×Ó×ŖŅʵďżÄæŗĶ·½Ļņ______________________”£
£Ø3£©ŌŚ20.00 mLµÄ0.1 mol”¤L£1 VO2+ČÜŅŗÖŠ£¬¼ÓČė0.195 gŠæ·Ū£¬Ē”ŗĆĶź³É·“Ó¦£¬Ōņ»¹Ō²śĪļæÉÄÜŹĒ______________________________________________________________”£
a£®V b£®V2£« c£®VO2+ d£®VO2£«
£Ø4£©ŅŃÖŖV2O5ÄÜŗĶŃĪĖį·“Ӧɜ³ÉĀČĘųŗĶVO2£«”£ĒėŌŁŠ“Ņ»øöĄė×Ó·“Ó¦·½³ĢŹ½£¬ĖµĆ÷»¹ŌŠŌ£ŗSO32-£¾Cl££¾VO2£«__________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Čõµē½āÖŹµÄµēĄėĘ½ŗā”¢ŃĪĄąµÄĖ®½āĘ½ŗāŗĶÄŃČÜĪļµÄČܽāĘ½ŗā¾łŹōÓŚ»ÆŃ§Ę½ŗā”£
¢ń.ŅŃÖŖH2AŌŚĖ®ÖŠ“ęŌŚŅŌĻĀĘ½ŗā£ŗH2A=H£«£«HA££¬HA£??H£«£«A2£”£
£Ø1£©³£ĪĀĻĀNaHAČÜŅŗµÄpH________(ĢīŠņŗÅ)£¬ŌŅņŹĒ_________________”£
A£®“óÓŚ7 B£®Š”ÓŚ7
C£®µČÓŚ7 D£®ĪŽ·ØČ·¶Ø
£Ø2£©Ä³ĪĀ¶ČĻĀ£¬ČōĻņ0.1 mol”¤L£1µÄNaHAČÜŅŗÖŠÖšµĪµĪ¼Ó0.1 mol”¤L£1KOHČÜŅŗÖĮČÜŅŗ³ŹÖŠŠŌ(ŗöĀŌ»ģŗĻŗóČÜŅŗµÄĢå»ż±ä»Æ)”£“ĖŹ±øĆ»ģŗĻČÜŅŗÖŠµÄĻĀĮŠ¹ŲĻµŅ»¶ØÕżČ·µÄŹĒ________”£
A£®c(H£«)”¤c(OH£)£½1.0”Į10£14
B£®c(Na£«)£«c(K£«)£½c(HA£)£«2c(A2£)
C£®c(Na£«)£¾c(K£«)
D£®c(Na£«)£«c(K£«)£½0.05 mol”¤L£1
£Ø3£©ŅŃÖŖ³£ĪĀĻĀH2AµÄøĘŃĪ(CaA)µÄ±„ŗĶČÜŅŗÖŠ“ęŌŚŅŌĻĀĘ½ŗā£ŗCaA(s)??Ca2£«(aq)£«A2£(aq)””¦¤H£¾0”£ČōŅŖŹ¹øĆČÜŅŗÖŠCa2£«ÅØ¶Č±äŠ”£¬æɲÉČ”µÄ“ėŹ©ÓŠ________”£
A£®ÉżøßĪĀ¶Č B£®½µµĶĪĀ¶Č
C£®¼ÓČėNH4Cl¾§Ģå D£®¼ÓČėNa2A¹ĢĢå
¢ņ.ŗ¬ÓŠCr2O72-µÄ·ĻĖ®¶¾ŠŌ½Ļ“ó£¬Ä³¹¤³§·ĻĖ®ÖŠŗ¬5.0”Į10£3 mol”¤L£1µÄCr2O72-”£ĪŖĮĖŹ¹·ĻĖ®µÄÅÅ·Å“ļ±ź£¬½ųŠŠČēĻĀ“¦Ąķ£ŗ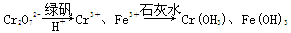
(1)øĆ·ĻĖ®ÖŠ¼ÓČėĀĢ·ÆŗĶH£«£¬·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________________”£
(2)Čō“¦ĄķŗóµÄ·ĻĖ®ÖŠ²ŠĮōµÄc(Fe3£«)£½2.0”Į10£13 mol”¤L£1£¬Ōņ²ŠĮōµÄCr3£«µÄÅضČĪŖ________”£
(ŅŃÖŖ£ŗKsp[Fe(OH)3]£½4.0”Į10£38£¬Ksp[Cr(OH)3]£½6.0”Į10£31)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(13·Ö)ĢśŗĶĢśµÄ»ÆŗĻĪļŌŚ¹¤ŅµÉś²śŗĶČÕ³£Éś»īÖŠ¶¼ÓŠ¹ć·ŗµÄÓĆĶ¾”£
£Ø1£©ŌŚ¶ØĻņ±¬ĘĘÖŠ£¬³£ĄūÓĆŃõ»ÆĢśÓėĀĮ·“Ó¦·Å³öµÄČČĮæĄ“ĒŠøīøÖ½ī£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ß£ß”£
£Ø2£©ŅŃÖŖ£ŗ2Fe2O3(s)£«3C(s)£½3CO2(g)£«4Fe(s) ”÷H£½+468.2 kJ”¤mol-1
C(s)+O2(g)£½CO2(g) ”÷H="-393.5" kJ”¤mol-1”£
ŌņFe(s)ÓėO2 (g)·“Ӧɜ³ÉFe2 O3 (s)µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ß_____________________”£
£Ø3£©æÉÓĆKMnO4ČÜŅŗµĪ¶ØFe2+µÄÅØ¶Č£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ČēĻĀ£ŗ5Fe2£«£«MnO4££«8H£«£½5Fe3£«£«Mn2£«£«4H2O
¢ŁKMnO4ČÜŅŗÓ¦Ź¢·ÅŌŚ£ß£ß£ß£ß£ßµĪ¶Ø¹ÜÖŠ£»
¢ŚÅŠ¶Ļ“ļµ½µĪ¶ØÖÕµćµÄĻÖĻóŹĒ£ß£ß£ß£ß£ß£»
¢ŪÓĆĮņĖįĖį»ÆµÄ0.020 00 mol”¤L-1”£KMnO4ČÜŅŗµĪ¶ØijFeSO4ČÜŅŗÖĮÖÕµć£¬ŹµŃ鏿¾Ż¼ĒĀ¼ČēĻĀ±ķ£ŗ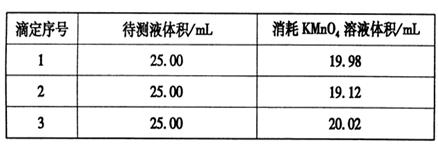
Ēė·ÖĪöŹż¾Ż²¢¼ĘĖć£¬øĆFeSO4ČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ£ß£ß£ß£ß£ß”£
£Ø4£©ŠĀŠĶÄÉĆײÄĮĻZnFe2Ox£¬æÉÓĆÓŚ³żČ„¹¤Ņµ·ĻĘųÖŠµÄijŠ©Ńõ»ÆĪļ”£ÖĘČ”ŠĀ²ÄĮĻŗĶ³żČ„·ĻĘųµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼£ŗ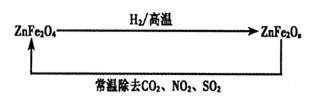
¢ŁŅŃÖŖZnFe2O4ÓėH2·“Ó¦µÄĪļÖŹµÄĮæÖ®±ČĪŖ2:1£¬ŌņZnFe2OxÖŠx=£ß£ß£ß£ß£ß£»
¢ŚÓĆZnFe2Ox³żČ„SO2µÄ¹ż³ĢÖŠ£¬Ńõ»Æ¼ĮŹĒ£ß£ß£ß£ß£ß”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĶŃĮņ¼¼ŹõÄÜÓŠŠ§æŲÖĘSO2¶ŌæÕĘųµÄĪŪČ¾”£
(1)ĻņĆŗÖŠ¼ÓČėŹÆ»ŅŹÆæɼõÉŁČ¼ÉÕ²śĪļÖŠSO2µÄŗ¬Į棬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ
_______________________________ӣ
(2)ŗ£Ė®³ŹČõ¼īŠŌ£¬Ö÷ŅŖŗ¬ÓŠNa£«”¢K£«”¢Ca2£«”¢Mg2£«”¢Cl£”¢SO42”Ŗ”¢Br£”¢HCO3”ŖµČ”£ŗ¬SO2µÄŃĢĘųæÉĄūÓĆŗ£Ė®ĶŃĮņ£¬Ę乤ŅÕĮ÷³ĢČēĶ¼ĖłŹ¾£ŗ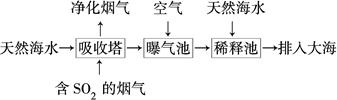
¢ŁĻņĘŲĘų³ŲÖŠĶØČėæÕĘųµÄÄæµÄŹĒ_____________________________________”£
¢ŚĶØČėæÕĘųŗóĘŲĘų³ŲÖŠŗ£Ė®ÓėĢģČ»ŗ£Ė®Ļą±Č£¬ÅضČÓŠĆ÷ĻŌ²»Ķ¬µÄĄė×ÓŹĒ________”£
a£®Cl£”””””” b£®SO42”Ŗ”””””” c£®Br£”””””” d£®HCO3”Ŗ
(3)ÓĆNaOHČÜŅŗĪüŹÕŃĢĘųÖŠµÄSO2£¬½«ĖłµĆµÄNa2SO3ČÜŅŗ½ųŠŠµē½ā£¬æɵƵ½NaOH£¬Ķ¬Ź±µĆµ½H2SO4£¬ĘäŌĄķČēĶ¼ĖłŹ¾(µē¼«²ÄĮĻĪŖŹÆÄ«)”£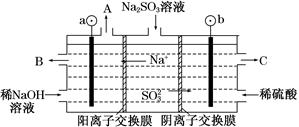
¢ŁĶ¼ÖŠa¼«ŅŖĮ¬½ÓµēŌ“µÄ________(Ģī”°Õż”±»ņ”°øŗ”±)¼«£¬CæŚĮ÷³öµÄĪļÖŹŹĒ________”£
¢ŚSO32”Ŗ·ÅµēµÄµē¼«·“Ó¦Ź½ĪŖ____________________________”£
¢Ūµē½ā¹ż³ĢÖŠŅõ¼«Ēų¼īŠŌĆ÷ĻŌŌöĒ棬ÓĆĘ½ŗāŅĘ¶ÆµÄŌĄķ½āŹĶŌŅņ£ŗ
__________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijĢ½¾æŠ”×齫Ņ»Åśµē×Ó·ĻĘśĪļ¼ņµ„“¦Ąķŗó£¬µĆµ½ŗ¬Cu”¢Al”¢Fe¼°ÉŁĮæAu”¢PtµČ½šŹōµÄ»ģŗĻĪļ£¬²¢Éč¼ĘČēĻĀÖʱøĮņĖįĶ¾§ĢåŗĶĪŽĖ®ĀČ»ÆĢśµÄ·½°ø£ŗ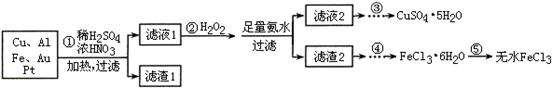
ŅŃÖŖ£ŗCu2+ + 4NH3”¤H2O£½[Cu(NH3)4]2+ + 4H2O
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©²½Öč¢ŁCuÓėĖį·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø2£©²½Öč¢Ś¼ÓH2O2µÄ×÷ÓĆŹĒ £¬ĀĖŌü2ĪŖ(Ģī»ÆѧŹ½) ”£
£Ø3£©²½Öč¢Ż²»ÄÜÖ±½Ó¼ÓČČĶŃĖ®µÄĄķÓÉŹĒ ”£
£Ø4£©ČōĀĖŅŗ1ÖŠCu2+µÄÅضČĪŖ0£®02mol”¤L-1£¬ŌņĒāŃõ»ÆĶæŖŹ¼³ĮµķŹ±µÄpH =
(ŅŃÖŖ£ŗKsp[Cu(OH)2]£½2£®0”Į10-20)”£
£Ø5£©ŅŃÖŖ£ŗ2Cu2+£«4I-£½ 2CuI”ż£«I2 I2£«2S2O32-£½ 2I-£«S4O62-
ijĶ¬Ń§ĪŖĮĖ²ā¶ØCuSO4”¤5H2O²śĘ·µÄÖŹĮæ·ÖŹżæÉ°“ČēĻĀ·½·Ø£ŗČ”3£®00g²śĘ·£¬ÓĆĖ®Čܽāŗ󣬼ÓČė×ćĮæµÄKIČÜŅŗ£¬³ä·Ö·“Ó¦ŗó¹żĀĖ”¢Ļ“µÓ£¬½«ĀĖŅŗĻ”ŹĶÖĮ250mL£¬Č”50mL¼ÓČėµķ·ŪČÜŅŗ×÷ÖøŹ¾¼Į£¬ÓĆ0£®080 mol”¤L-1 Na2S2O3±ź×¼ČÜŅŗµĪ¶Ø£¬“ļµ½µĪ¶ØÖÕµćµÄŅĄ¾ŻŹĒ ”£
ĖÄ“ĪĘ½ŠŠŹµŃéŗÄČ„Na2S2O3±ź×¼ČÜŅŗŹż¾ŻČēĻĀ£ŗ
| ŹµŃéŠņŗÅ | 1 | 2 | 3 | 4 |
| ĻūŗÄNa2S2O3±ź×¼ČÜŅŗ(mL) | 25£®00 | 25£®02 | 26£®20 | 24£®98 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¹żŃõ»ÆĒāĖ®ČÜŅŗĖ׳ĘĖ«ŃõĖ®£¬·Šµć±ČĖ®øߣ¬Óö¹ā”¢ČČ¼°ÖŲ½šŹō»ÆŗĻĪļµČ¾łÄÜŅżĘš·Ö½ā”£
£Ø1£©Ä³ŹŌ¼Į³§ĻČÖʵĆ7%~8%µÄĖ«ŃõĖ®£¬Óū½«ĘäÅØĖõ³É30%µÄČÜŅŗ£¬ŹŹŅĖ·½·ØŹĒ
£ØĢīŠ“±ąŗÅ£©”£
a£®³£Ń¹ÕōĮó ”” b£®¼õŃ¹ÕōĮó c£®¼ÓČėÉśŹÆ»Ņ³£Ń¹ÕōĮó ”””” d£®¼ÓŃ¹ÕōĮó
£Ø2£©Čē¹ūµĆµ½µÄĖ«ŃõĖ®ÖŠŃõŌŖĖŲµÄŗ¬ĮæĪŖ90%£¬Ōņ¹żŃõ»ÆĒāµÄ“æ¶ČĪŖ ”£ÖŚĖłÖÜÖŖ£¬ĒāĘųŌŚæÕĘųÖŠČ¼ÉÕÉś³ÉĖ®”£ÓŠČĖĢį³ö£¬ĒāĘųŌŚæÕĘųÖŠČ¼ÉÕŅ²æÉÄÜÉś³ÉH2O2£¬µ«ĖüŅņøßĪĀ¶ų·Ö½āĮĖ”£ĪŖĮĖŃéÖ¤ĒāĘųŌŚæÕĘųÖŠČ¼ÉյIJśĪļÖŠŹĒ·ńŗ¬ÓŠH2O2£¬Ä³æĪĶāŠ”×éĶ¬Ń§Éč¼ĘµÄŹµŃé×°ÖĆ¼ūĶ¼-1”£
£Ø3£©¼×Ķ¬Ń§Ļė“ÓĻĀĶ¼-2µÄ¢Ł£¢Ü֊єȔĢę“śĶ¼£1·½æņÖŠµÄ×°ÖĆ£¬æÉŠŠµÄŹĒ £ØĢīŠ“±ąŗÅ£©”£
£Ø4£©ČōŅŅĶ¬Ń§ÓĆĖįŠŌøßĆĢĖį¼ŲČÜŅŗ¼ģ²āµ½ĮĖH2O2µÄ“ęŌŚ£¬Ķź³ÉøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
”ś + ”” Mn2+ + H2O
±ūĶ¬Ń§¶ŌŅŅµÄ¼ģŃé·½°øĢį³öĮĖÖŹŅÉ£ŗČōŠæĮ£ÓėĻ”ĮņĖįµÄ·“Ó¦ÖŠ²śÉśĮĖÉŁĮæH2SµČ»¹ŌŠŌĘųĢ壬Ņ²»įŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«”£Ēė¶ŌŅŅĶ¬Ń§µÄŹµŃé·½°øĢį³öøĽų½ØŅé£ŗ ”£
£Ø5£©¹żĢ¼ĖįÄĘ£Ø2Na2CO3?3H2O2£©Ė×³Ę¹ĢĢåĖ«ŃõĖ®£¬¼«Ņ×·Ö½ā£¬Ęä·Ö½ā·“Ó¦µÄ»Æѧ·½³ĢŹ½æɱķŹ¾ĪŖ£ŗ2 (2Na2CO3?3H2O2) ”ś 4Na2CO3 + 6H2O + 3O2”ü
Č”Ņ»¶ØĮæµÄ¹żĢ¼ĖįÄĘŌŚĆܱÕČŻĘ÷ÖŠŹ¹ĖüĶźČ«·Ö½ā£¬²āµĆÉś³ÉŃõĘų12.0g”£ĄäČ“µ½ŹŅĪĀŗó£¬ĻņĖłµĆ²śĪļÖŠ¼ÓĖ®ÅäÖĘ³É10.6% µÄNa2CO3ČÜŅŗ£¬Šč¼ÓĖ® g”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com