
(1)ʵ���в�������������������������(�����ʽ)��?
(2)ԭ��Һ��һ�����е�������������������������������������
���ܺ��е���������������������֤�����Ӵ��ڵķ����ǣ�������������������������������
(3)ԭ��Һ��һ�������е�������������������������?
(4)�ڳ����м�������NaOH��Һ�����������ܽ⣬������Ӧ�����ӷ���ʽΪ��_________________��
 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| O | - 3 |
| O | 2- 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
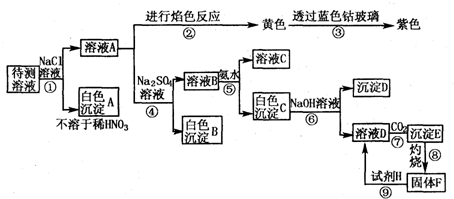
| A | B | C | E |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ʵ��ٿɵó��Ľ����ǣ�ԭ��Һ�к�S2O32-����Fe3+��NO3- | B��ԭ��Һ�п϶����ڵ������ǣ�S2O32-��NH4+��Mg2+��SO42- | C������ȷ���Ƿ���ڵ������ǣ�HCO3-��SO42- | D��ʵ��۵õ���ɫ����ΪBaSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ����Һ�п��ܺ������������е�һ�ֻ��֣�Na+��Mg2+��Cu2+��OH-��Cl-��![]() ��
��![]() ���ֽ�������ʵ�飺
���ֽ�������ʵ�飺
��1���ú�ɫʯ����ֽ���飬��ֽ����ɫ��
��2����ȡ������Һ��εμ�������������������ų����ټ���BaCl2��Һ��û�г������ɡ�
��3����ȡ������Һ�������ữ����AgNO3��Һ�а�ɫ�������ɡ�
��������ʵ���ƶϣ�ԭ��Һ�п϶���_______________���ӣ��϶�û��________________���ӣ����ܿ϶�����______________���ӡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com