
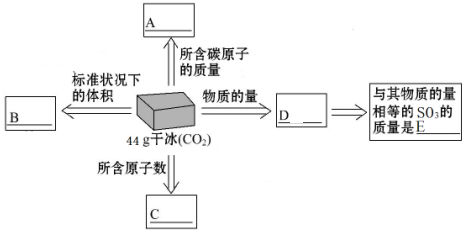
���� ��1������n=$\frac{m}{M}$����44g�ɱ������ʵ���������V=nVm��������ռ�е����������ԭ�����ʵ���Ϊ�ɱ���3�����ٸ���N=nNA���㺬��ԭ����Ŀ��̼ԭ�����ʵ������ڸɱ������ʵ���������m=nM���㺬��̼ԭ�ӵ��������������������ͷ���ʵ�����ȣ�����m=nM�������������������
��2������n=$\frac{V}{{V}_{m}}$����HCl�����ʵ������ٸ���c=$\frac{n}{V}$����������Һ���ʵ���Ũ�ȣ�
��3������ϡ�Ͷ��ɼ�����Ҫ1.25mol•L-1��NaOH��Һ�������
��4������M=$\frac{m}{n}$����MgX2��Ħ��������
��� �⣺��1��D.44g�ɱ������ʵ���Ϊ��$\frac{44g}{44g/mol}$=1mol��
B����״���¸ö�����̼ռ�е����Ϊ��1mol��22.4L/mol=22.4L��
C������ԭ�����ʵ���Ϊ�ɱ���3��������ԭ����Ŀ=1mol��3��6.02��1023mol-1=1.806��1024��
A������Cԭ���غ��n��C��=n��CO2����Cԭ�ӵ�����m=nM=1mol��12g/mol=12g��
E�����������������̼�����ʵ�����ȣ�����������������m=nM=1mol��64g/mol=64g��
�ʴ�Ϊ��12g��22.4L��1.806��1024��1mol��64g��
��2��n��HC��=$\frac{22.4L}{22.4L/mol}$=1mol��c��HCl��=$\frac{1mol}{0.5L}$=2mol/L��
�ʴ�Ϊ��2mol/L��
��3��1L 0.5mol•L-1NaOH�����ƹ������������Ƶ����ʵ������䣬����Ҫ1.25mol•L-1��NaOH��Һ�����=$\frac{0.5mol/L��1L}{1.25mol/L}$=0.4L=400mL��
�ʴ�Ϊ��400��
��4��19g MgX2����Mg2+0.2mol������Mgԭ���غ�ã�n��MgX2��=n��Mg ��=0.2mol����MgX2��Ħ������Ϊ��M��MgX2��=$\frac{19g}{0.2mol}$=95g/mol��
�ʴ�Ϊ��95g/mol��
���� ���⿼�������ʵ������йؼ��㣬��Ŀ�Ѷ��еȣ���ȷ�й����ʵ����Ļ�����ʽ�ǽⱾ��ؼ����ٽ�ϸ���������֮��Ĺ�ϵʽ�������������������ѧ���ķ�����������ѧ����������


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Al2O3 | B�� | MgO | C�� | SiO2 | D�� | Fe2O3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ͼױ��ж����м����ױ����Ա�����KMnO4��Һ�����ɱ����ᣬ�����鲻�ܱ���������˵�������Բ���������Ӱ�� | |
| B�� | ����Ũ���ᡢŨ����������100��110��������ɶ������������ױ���100��ʱ���������������ױ���˵�����Ա���������Ӱ�� | |
| C�� | ú�к��б��ͼױ����������ȸ�������ķ��������Ƿ������ | |
| D�� | ��ȥ���л���������ױ��ɼ�������������KMnO4��Һ����ַ�Ӧ���ټ���������NaOH��Һ��Ȼ���Һ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ${\;}_{83}^{209}$Bi ��${\;}_{83}^{210}$Bi������83������ | |
| B�� | ͬλ�أ�H2��D2��T2 | |
| C�� | H2O��NH3�����о�����ͬ���������͵����� | |
| D�� | ϡ�������ԭ������㶼�ﵽ8�����ȶ��ṹ���ʶ������������ʷ�����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ��Ӧ���ʱ��뷴Ӧ��;���й� | |
| B�� | �����£�ϡ��0.1mol•L-1CH3COOH��Һ����Һ�ĵ����������� | |
| C�� | ���³�ѹ�£�22.4L Cl2�к��еķ�����Ϊ6.02��1023�� | |
| D�� | ��������ͭ��a��b����;����ȫת��ΪCu��NO3��2��;��a��b���ĵ�����һ����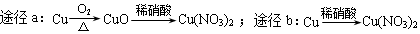 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaHCO3��Һ�У�c��Na+��=c��CO32-��+c��HCO3-��+c��H2CO3�� | |
| B�� | ��c��H+��=1��10-13mol•L-1����Һ�У�Na+��Fe3+��Cl-��SO42-�ܴ������� | |
| C�� | ͭ����FeCl3��Һ�У�Cu+Fe3+=Fe2++Cu2+ | |
| D�� | ����Һ�м���BaCl2��Һ���ټ����ᣬ�а�ɫ������˵������Һ��һ������SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�켪��ʡ�����ϵڶ���ģ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
��1.52 gͭþ�Ͻ���ȫ�ܽ���50 mL�ܶ�Ϊ1.40 g��mL��1����������Ϊ63%��Ũ�����У��õ�NO2��N2O4�Ļ������ 1120 mL����״��������Ӧ�����Һ�м���1.0 mol��L��1NaOH��Һ������������ȫ������ʱ���õ�2.54 g����������˵������ȷ����
A���úϽ���ͭ��þ�����ʵ���֮����1��2
B����Ũ������HNO3�����ʵ���Ũ����14.0 mo l��L��1
l��L��1
C��NO2��N2O4�Ļ�������У�NO2�����������80%
D���õ�2.54 g����ʱ������NaOH��Һ�������640 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡɽһ�����ϵڶ���ͳ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
һ�����ʵ�����п��ϡHNO3��Ӧ��������Ļ�ԭ����ΪN2O����Ӧ������п��ʣ�࣬��μӷ�Ӧ��HNO3�б���ԭ��HNO3��δ����ԭ��HNO3�����ʵ���֮���ǣ� ��
A��1��4 B��1��5 C��2��3 D��2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com