
| A��Ư����Һ�ڿ�����ʧЧ��ClO����CO2��H2O=HClO��HCO3- |
B����Ũ������MnO2��Ӧ��ȡ����������MnO2��2H����2Cl�� Mn2����Cl2����2H2O Mn2����Cl2����2H2O |
| C����NaAlO2��Һ��ͨ�����CO2��Al(OH)3��AlO2-��CO2��2H2O=Al(OH)3����HCO3- |
| D����ǿ����Һ�д���������Fe(OH)3��Ӧ����Na2FeO4��3ClO����2Fe(OH)3=2FeO42-��3Cl����H2O��4H�� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.01mol��L��1NH4Al(SO4)2��Һ��0.02mol��L��1Ba(OH)2��Һ�������ϣ� NH4��+Al3��+2SO42��+2Ba2��+4OH��=2BaSO4��+Al(OH)3��+NH3��H2O |
| B��һ��������,��0.5mol N2(g)��1.5molH2(g)�����ܱյ������г�ַ�Ӧ����NH3(g),����19.3kJ�����Ȼ�ѧ����ʽΪ�� N2(g)+3H2(g) 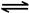 2NH3(g)��H=-38.6kJ��mol-1 2NH3(g)��H=-38.6kJ��mol-1 |
| C���������е���AgNO3��Һ�������е���Ԫ�أ�Br-+Ag+==AgBr�� |
| D��CO(g)��ȼ������283.0 kJ��mol��1����CO2�ֽ���Ȼ�ѧ����ʽΪ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ���ӷ���ʽ | ���� |
| A | AgCl(s) + I-( aq)  AgI(s) + Cl-(aq) AgI(s) + Cl-(aq) | �ܽ�ȣ�AgI > AgCl |
| B | Fe2��+ H2O2 +2H��= Fe3��+2H2O | �����ԣ�H2O2 > Fe3�� |
| C | CO32- + CO2 + H2O = 2HCO3- | �ȶ��ԣ�HCO3- > CO32- |
| D | NH3 + H3O+=NH4++ H2O | ������������NH3> H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ��H������CO2����Cr3����H2O�����������ʽ��ƽ��H���Ļ�ѧ������Ϊ
��H������CO2����Cr3����H2O�����������ʽ��ƽ��H���Ļ�ѧ������Ϊ| A��10 |
| B��12 |
| C��14 |
| D��16 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ba(OH)2��Һ�еμ�NaHSO4��Һ�����ԣ�Ba2++H++OH��+SO42��=BaSO4��+H2O |
| B��Cl2ͨ��ˮ�У�Cl2��H2O=2H����Cl����ClO�� |
| C���Ȼ�����Һ�м��������ˮ��Al3����4NH3��H2O=AlO2-��4NH4+ |
| D���Ȼ�������Һ��ͨ��������2Fe2����Cl2=2Fe3����2Cl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A����ⱥ��ʳ��ˮ��2Cl��+ 2H2O 2OH��+ H2��+ Cl2�� 2OH��+ H2��+ Cl2�� |
| B�������ȥˮ���е�CaCO3��CaCO3+ 2H+��Ca2+ + H2O + CO2�� |
C��Ư����Һ�ڿ�����ʧЧ��ClO��+ CO2 + H2O��HClO + HCO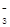 |
D����NaHSO4��Һ�еμ�NaHCO3��Һ��HSO + HCO + HCO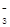 �� H2O + SO �� H2O + SO + CO2�� + CO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��AlCl3��Һ�м��������İ�ˮ��Al3++ 3OH�� �T Al(OH)3�� |
| B������������Һ��ϡ H2SO4��Ba2++OH��+SO42��+H+ �TBaSO4��+H2O |
| C�����Ȼ�������Һ��ͨ��������2Fe2++Cl2�T2Fe3++2Cl�� |
| D����������ͨ���廯������Һ��2Fe2����2Br����2Cl2�TFe3����Br2��4Cl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com