
 A��B��C��D��E������Һ�ֱ���NaOH��NH3•H2O��CH3COOH��HCl��NH4HSO4�е�һ�֣������½�������ʵ�飺
A��B��C��D��E������Һ�ֱ���NaOH��NH3•H2O��CH3COOH��HCl��NH4HSO4�е�һ�֣������½�������ʵ�飺���� �����к���������Aֻ��NaOH��NH3•H2O�����ʵ�����A������ʵ���Ũ��B��D��ϳ����ԣ�D�������٣�˵��D���Ա�B�ļ���ǿ������D��NaOH��BΪNH3•H2O������Ũ�Ⱦ�Ϊ0.1mol•L-1C��D��Һ�������ϣ���Һ�����ԣ���CΪNH4HSO4������Ũ�Ⱦ�Ϊ0.1mol•L-1A��E��Һ��pH��A��E����AΪHCl��EΪCH3COOH��
��1���������Ϸ����жϣ�DΪ�������ơ�EΪ���
��2���١�B��ҺΪ��ˮ��Һ����NH3•H2O?OH-+NH4+��֪����ˮ�ٽ����룬��n��NH3•H2O�����٣�n��OH-������
�ڡ���NH3•H2O?OH-+NH4+��֪����ˮ�ٽ����룻
�ۡ�Kwֻ���¶��йأ�
�ܡ���ˮ�ٽ����룻
��3���ٷ�Ӧ����Ҫ��ʱ�����̣�
�ڿ�ʼ������Ũ����ͬ����Ӧʱ��������ͬ��
������������ͬ����ϵ����غ㣬�μӷ�Ӧ��п�۵����ʵ�����
�ܷ�Ӧ���̵�ƽ�����ʣ�����������
�����ݢݷ�����֪E��Һ����п��ʣ�ࣻ
��4��������������ʵ���Ũ��B��C��Ϻ�õ���NH4��2SO4��Һ��NH4+ˮ����Һ�����ԣ�PH��7��
��5�������£���0.01mol/L C��NH4HSO4����Һ�еμ�0.01mol/L D��NaOH����Һ�����ԣ�NH4HSO4�е���NaOH��Һ��NaOH������NH4HSO4�������H+���ã���ΪH+���OH-��������NH4+���OH-������ǿ��ԭ���Dz���H2O��NH3•H2O���ѵ��룩���������Ħ����NaOHʱ�����ý�H+�кͣ���ʱc��Na+��=c��SO42-��������ʱ��Һ�л���NH4+��NH4+ˮ��ʹ��Һ�����ԣ����Ҫʹ��Һ�����ԣ������������NaOH����Ȼ������ʱc��OH-��=c��H+����
��� �⣺��1���к���������Aֻ��NaOH��NH3•H2O�����ʵ�����A������ʵ���Ũ��B��D��ϳ����ԣ�D�������٣�˵��D���Ա�B�ļ���ǿ������D��NaOH��EΪCH3COOH���ʴ�Ϊ��NaOH��CH3COOH��
��2������NH3•H2O?OH-+NH4+��֪����ˮ�ٽ����룬��n��NH3•H2O�����٣�n��OH-��������$\frac{c��N{H}_{3}•{H}_{2}O��}{c��O{H}^{-}��}$=$\frac{n��N{H}_{3}•{H}_{2}O��}{n��O{H}^{-}��}$��С���ʢٴ���
����NH3•H2O?OH-+NH4+��֪����ˮ�ٽ����룬��n��NH3•H2O�����٣�n��OH-������c��NH3•H2O����c��OH-������С��c��H+����С����$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$��С���ʢ���ȷ��
�����ˮϡ��ʱ���¶Ȳ��䣬��c��H+����c��OH-���ij˻����䣬�ʢ۴���
����NH3•H2O?OH-+NH4+��֪����ˮ�ٽ����룬OH-�����ʵ������ʢܴ���
�ʴ�Ϊ���٢ڣ�
��3���ٸ���������п��Ӧ�õ����������ŷ�Ӧ�Ľ��У�CH3COOH���ϵ����H+����Ӧ���ʱ������п죬����������һ���࣬��Ӧ����Ҫ��ʱ��HCl��CH3COOH���ʢٴ���
�ڸ���������п��Ӧ�õ���������ʼ��Һ��c��H+����ȣ���Ӧʱ������HCl=CH3COOH���ʢڴ���
�۸���������п��Ӧ�õ���������������һ����Һ�д���п���ų�������������ͬ��˵��������пʣ�࣬CH3COOH��п��ȫ��Ӧ���μӷ�Ӧ��п�����ʵ�����ȣ��ʢ���ȷ��
�ܸ���������п��Ӧ�õ����������ŷ�Ӧ�Ľ��У�CH3COOH���ϵ����H+����Ӧ���ʱ������п죬�ʢ���ȷ��
�ݸ���������п��Ӧ�õ���������������һ����Һ�д���п���ų�������������ͬ��˵��������пʣ�࣬CH3COOH��п��ȫ��Ӧ���μӷ�Ӧ��п�����ʵ�����ȣ��ʢݴ���
�ʴ�Ϊ���ۢܣ�
��4��������������ʵ���Ũ��NH3•H2O����NH4HSO4��Ϻ���Һ������Ӧ��NH3•H2O+NH4HSO4=��NH4��2SO4+H2O��NH4��2SO4Ҫ����ˮ�⣺NH4++H2O?NH3•H2O+H+����Һ�����ԣ�����ˮ��ƽ�������ƶ���c��H+������pH��С���ʴ�Ϊ���ܣ�
��5��NH4HSO4�е���NaOH��Һ��NaOH������NH4HSO4�������H+���ã���ΪH+���OH-��������NH4+���OH-������ǿ��ԭ���Dz���H2O��NH3•H2O���ѵ��룩���������Ħ����NaOHʱ�����ý�H+�кͣ���ʱc��Na+��=c��SO42-��������ʱ��Һ�л���NH4+��NH4+ˮ��ʹ��Һ�����ԣ����Ҫʹ��Һ�����ԣ������������NaOH����Ȼ������ʱc��OH-��=c��H+����c��Na+����c��SO42- ����c��NH4+����c��OH-��=c��H+������
�ʴ�Ϊ��c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
���� ���⿼��������Һ֮��ķ�Ӧ���漰��ǿ����ʡ�������ʵĵ��롢�����ˮ�⼰��Һ��pHֵ������Ũ�ȴ�С�ıȽϵ�֪ʶ����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
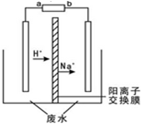 2016������3��2���ڱ��������Ļ�����漰�����������ڶ���У���ˮ��Դ�ı����ͺ������á���Ȼ������Ⱥ�ڹ�ע�Ľ��㣮�����о����֣��ø�Ĥ��ⷨ������Ũ����ȩ��ˮ���й������̼���Ľϵ͵��ŵ㣬��ԭ����ʹ��ȩ�ֱ�����������������Ӧ��ת��Ϊ�Ҵ������ᣬ�ܷ�Ӧ���£�2CH3CHO+H2O$\frac{\underline{\;���\;}}{\;}$CH3CH2OH+CH3COOHʵ�����У���һ��Ũ�ȵ���ȩ-Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ�Ĵ������̣���װ��ʾ��ͼ��ͼ��ʾ�������й�˵�����������ǣ�������
2016������3��2���ڱ��������Ļ�����漰�����������ڶ���У���ˮ��Դ�ı����ͺ������á���Ȼ������Ⱥ�ڹ�ע�Ľ��㣮�����о����֣��ø�Ĥ��ⷨ������Ũ����ȩ��ˮ���й������̼���Ľϵ͵��ŵ㣬��ԭ����ʹ��ȩ�ֱ�����������������Ӧ��ת��Ϊ�Ҵ������ᣬ�ܷ�Ӧ���£�2CH3CHO+H2O$\frac{\underline{\;���\;}}{\;}$CH3CH2OH+CH3COOHʵ�����У���һ��Ũ�ȵ���ȩ-Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ�Ĵ������̣���װ��ʾ��ͼ��ͼ��ʾ�������й�˵�����������ǣ�������| A�� | ����п-�̼��Ե��Ϊֱ����Դ����b������ΪZn | |
| B�� | ��֪��ȩ���Ҵ��ķе�ֱ�Ϊ20.8�桢78.4�棬�ӵ������Һ�з�����Ҵ��ֲ�Ʒ�ķ��������� | |
| C�� | ����Ӧ������ÿ����1mol�Ҵ���������������������11.2L | |
| D�� | �������У����������������⣬���������·�Ӧ��CH3CHO+2H2O-2e-=CH3COOH+2H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ��������ڸ���NH3��H2��O2������ | |
| B�� | �������������Ư��ֽ����ë��˿�� | |
| C�� | �������Ӽ��Ļ�����һ�������ӻ����� | |
| D�� | �ɱ�����û�ж��ѻ�ѧ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | B��D��C��A | B�� | C��A��B��D | C�� | A��C��D��B | D�� | A��B��C��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �۵㣺ʯӢ��ʳ�Σ������ɱ� | |
| B�� | ������ӣ�H+��������OH-��HCO-3��CH3COO- | |
| C�� | ���Ӱ뾶��S2-��Cl-��Al3+��O2- | |
| D�� | ���ȶ��ԣ�HF��HCl��H2S��PH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 6��3��2 | B�� | 1��2��3 | C�� | 3��6��2 | D�� | 2��1��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol Fe�������ķǽ�����Ӧת�Ƶ���Ϊ2mol | |
| B�� | 1mol Fe�����������ᷴӦת�Ƶ���Ϊ3mol | |
| C�� | 1mol FeCl3������Zn��Ӧת�Ƶ���Ϊ2mol | |
| D�� | 1mol FeCl2������Cu��Ӧת�Ƶ���Ϊ2mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com