
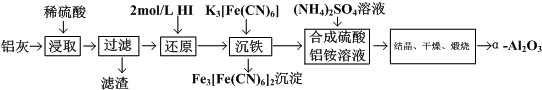
| A�� | ���Ƚ������������ҵĽ�ȡ�� | |
| B�� | �����е�HI��������H2O2���� | |
| C�� | �����е�K3[Fe��CN��6]��������NaOH��Һ���� | |
| D�� | �����еĵõ�������������ľ��巽��Ϊ�������ᾧ�����ȹ��ˡ�ϴ�ӡ����� |
���� ���ҵ���Ҫ�ɷ�ΪAl2O3������������SiO2��FeO��Fe2O3����ϡ���ᣬAl2O3��FeO��Fe2O3ת��Ϊ���ӣ�SiO2���������ᣬ���ˣ���Һ�к���Al3+��Fe2+��Fe3+���ӵ⻯�⣬��Fe3+��ԭΪFe2+������K3[Fe��CN��6]��Fe2+ת��Ϊ���������ˣ�����Һ�м�������泥�����NH4Al��SO4��2���ᾧ��������յõ���-Al2O3���ݴ˴��⣮
��� �⣺���ҵ���Ҫ�ɷ�ΪAl2O3������������SiO2��FeO��Fe2O3����ϡ���ᣬAl2O3��FeO��Fe2O3ת��Ϊ���ӣ�SiO2���������ᣬ���ˣ���Һ�к���Al3+��Fe2+��Fe3+���ӵ⻯�⣬��Fe3+��ԭΪFe2+������K3[Fe��CN��6]��Fe2+ת��Ϊ���������ˣ�����Һ�м�������泥�����NH4Al��SO4��2���ᾧ��������յõ���-Al2O3��
A�����Ƚ������������ҵĽ�ȡ�ʣ���A��ȷ��
B�������е�HI������ԭ�����������������ӣ���������H2O2���棬��B����
C���������������������ƿ����������������������������������ƴ���K3[Fe��CN��6]�����������Ԫ�ص���ʧ����C����
D������Һ�еõ�������茶���ľ��巽��Ϊ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ������D����
��ѡ��A��
���� ��������������ȡ��������Ϊ���壬����Ԫ�ػ��������ʼ��ת����������ԭ��ʵ�����������֪ʶ�㣬ע������Ʊ�ԭ�����������ʵ������Լ���ط�Ӧ����ʽ����д����Ŀ�Ѷ��еȣ�


 ����5��2���ϵ�д�
����5��2���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ֱ�μ���ͬ���ʵ���Ũ�ȵ����ᣬ����CO2�����ʣ�Na2CO3��NaHCO3 | |
| B�� | �ֱ������Һ���ټ������ʯ��ˮ���ް�ɫ�������ɵ���NaHCO3 | |
| C�� | �ֱ����������Ʒ��û�в������ʵ���NaHCO3 | |
| D�� | ������һ�������²����ת�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���е�ˮ�ĵ�����Һ10ml�ֳ����ȷ�����֧�Թܣ��ֱ����ϡ�������Һ1ml����ǰ����ɫ�� | |
| B�� | �����£���ʢ��NO2��N2O4��ƽ����ϵ��ѹ����ϵ��ɫ���ձ�dz | |
| C�� | ������Һ������KMnO4��Һ��Ϻ�����������Է�Ӧ�д����ã���ʹ��Ӧ���ʼӿ� | |
| D�� | ��K2Cr2O7��Һ�еμ�Ũ���ᣬ��Һ��ɫ�ɳ�ɫ���ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
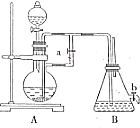 ��ͼ��ʾװ�ã���������ȡ�۲�Fe��OH��2�ڿ����б���������ɫ�仯��ʵ��ʱ����ʹ����м��6mol/L�����ᣬ�����Լ���ѡ��
��ͼ��ʾװ�ã���������ȡ�۲�Fe��OH��2�ڿ����б���������ɫ�仯��ʵ��ʱ����ʹ����м��6mol/L�����ᣬ�����Լ���ѡ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CS2��SO3 | B�� | P4��CH4 | C�� | NH3��H2S | D�� | CCl4��C2H4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH3+HCl�TNH4Cl | B�� | 4NH3+5O2 $\frac{\underline{\;\;��\;\;}}{\;}$ 4NO+6H2O | ||
| C�� | 3CuO+2NH3 $\frac{\underline{\;\;��\;\;}}{\;}$ 3Cu+N2+3H2O | D�� | 8NH3+3Cl2 $\frac{\underline{\;\;��\;\;}}{\;}$ 6NH4Cl+N2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�22.4 L H2O ������Ϊ18 g | |
| B�� | 0.5 mol O2�к��еķ�����ԼΪ6.02��1023 | |
| C�� | 0.1 mol/L Na2CO3��Һ�к�Na+�����ʵ���Ϊ0.1 mol | |
| D�� | ���³�ѹ�£�1.7 g NH3���е�������ԼΪ6.02��1023 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com