
 Na2S2O3Ė×³Ę“óĖÕ“ņ£Øŗ£²Ø£©ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£®ÓĆNa2SO3ŗĶĮņ·ŪŌŚĖ®ČÜŅŗÖŠ¼ÓČČ·“Ó¦£¬æÉŅŌÖʵĆNa2S2O3£®ŅŃÖŖ10”ęŗĶ70”ꏱ£¬Na2S2O3ŌŚĖ®ÖŠµÄČܽā¶Č·Ö±šĪŖ60.0gŗĶ212g£®³£ĪĀĻĀ£¬“ÓČÜŅŗÖŠĪö³öµÄ¾§ĢåŹĒNa2S2O3•5H2O£®
Na2S2O3Ė×³Ę“óĖÕ“ņ£Øŗ£²Ø£©ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£®ÓĆNa2SO3ŗĶĮņ·ŪŌŚĖ®ČÜŅŗÖŠ¼ÓČČ·“Ó¦£¬æÉŅŌÖʵĆNa2S2O3£®ŅŃÖŖ10”ęŗĶ70”ꏱ£¬Na2S2O3ŌŚĖ®ÖŠµÄČܽā¶Č·Ö±šĪŖ60.0gŗĶ212g£®³£ĪĀĻĀ£¬“ÓČÜŅŗÖŠĪö³öµÄ¾§ĢåŹĒNa2S2O3•5H2O£®| µĪ¶Ø“ĪŹż | µĪ¶ØĒ°¶ĮŹż£ØmL£© | µĪ¶ØµĪ¶Øŗó¶ĮŹż£ØmL£© |
| µŚŅ»“Ī | 0.00 | 30.82 |
| µŚ¶ž“Ī | 0.00 | 30.80 |
| µŚČż“Ī | 0.00 | 30.78 |
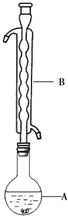
·ÖĪö £Ø1£©øł¾Ż×°ÖĆĶ¼æÉÖŖ£¬ŅĒĘ÷BĪŖĒņŠĪĄäÄż¹Ü£¬½įÉÕĘæÖŠµÄŅŗĢå½ųŠŠĄäÄż»ŲĮ÷£¬Įņ·ŪÄŃČÜÓŚĖ®”¢Ī¢ČÜÓŚŅŅ“¼£¬ŅŅ“¼ŹŖČóæÉŅŌŹ¹Įņ·ŪŅ×ÓŚ·ÖÉ¢µ½ČÜŅŗÖŠ£¬ĮņŌŚ¾Ę¾«ÖŠĪ¢ČÜ£¬æÉŅŌŌö“ó½Ó“„Ć껿£¬Ģįøß·“Ó¦ĖŁĀŹ£»
£Ø2£©ČÜŅŗÖŠµĆµ½ČÜÖŹ¹ĢĢåµÄ·½·ØŹĒĶعżÕō·¢ÅØĖõ£¬ĄäČ“½į¾§£¬¹żĀĖĻ“µÓ£¬øÉŌļµČ²½ÖčµĆµ½£»
£Ø3£©ÓÉÓŚS2O32?¾ßÓŠ»¹ŌŠŌ£¬Ņ×±»ŃõĘųŃõ»Æ³ÉĮņĖįøłĄė×ÓæÉÖŖŌÓÖŹĪŖĮņĖįÄĘ£¬ŅĄ¾ŻĮņĖįøłĄė×Ó¼ģŃé·½Éč¼ĘŹµŃé¼ģŃ飻
£Ø4£©µāĖ®ÓŠŃõ»ÆŠŌ£¬ÄÜøÆŹ“Ļš½ŗ¹Ü£»ŅĄ¾Ż»Æѧ·“Ó¦·½³ĢŹ½2S2O32-+I2ØTS4O42-+2I-¼ĘĖć¼“æÉ£¬²ā¶ØѳʷµÄ“æ¶Č¾ĶŹĒŅŌµā±ź×¼ČÜŅŗĪŖ±ź×¼µÄ£¬Čē¹ūµĪ¶ØÖÕµćƻӊæŲÖĘŗĆ£¬µā±ź×¼ČÜŅŗµĪ¼Ó¹żĮæŅ²»įŗĶŃĒĮņĖįÄĘ·“Ó¦£®
½ā“š ½ā£ŗ£Ø1£©øł¾Ż×°ÖĆĶ¼æÉÖŖ£¬ŅĒĘ÷BĪŖĒņŠĪĄäÄż¹Ü£¬½įÉÕĘæÖŠµÄŅŗĢå½ųŠŠĄäÄż»ŲĮ÷£¬Įņ·ŪÄŃČÜÓŚĖ®Ī¢ČÜÓŚŅŅ“¼£¬ĖłŅŌĮņ·ŪŌŚ·“Ó¦Ē°ÓĆŅŅ“¼ŹŖČóŹĒŹ¹Įņ·ŪŅ×ÓŚ·ÖÉ¢µ½ČÜŅŗÖŠ£¬ÓŠĄūÓŚĮņ·ŪŗĶNa2SO3ČÜŅŗ³ä·Ö½Ó“„£¬¼Óæģ·“Ó¦ĖŁĀŹ
¹Ź“š°øĪŖ£ŗĒņŠĪĄäÄż¹Ü£»ĄäÄż»ŲĮ÷£»Ōö¼Ó·“Ó¦Īļ½Ó“„Ć껿£¬Ģįøß·“Ó¦ĖŁĀŹ£»
£Ø2£©ĶعżÕō·¢ÅØĖõ£¬ĄäČ“½į¾§£¬¹żĀĖĻ“µÓ£¬øÉŌļµČ²½ÖčµĆµ½ČÜŅŗÖŠµÄČÜÖŹ¹ĢĢ壬
¹Ź“š°øĪŖ£ŗÕō·¢ÅØĖõ£»ĄäČ“½į¾§£»
£Ø3£©S2O32?¾ßÓŠ»¹ŌŠŌ£¬Äܹ»±»ŃõĘųŃõ»Æ³ÉĮņĖįøłĄė×Ó£¬ĀĖŅŗÖŠ³żNa2S2O3ŗĶæÉÄÜĪ“·“Ó¦ĶźČ«µÄNa2SO3Ķā£¬“ęŌŚ±»Ńõ»Æ²śĪļĮņĖįÄĘ£¬ĖłŅŌæÉÄÜ“ęŌŚµÄŌÓÖŹŹĒĮņĖįÄĘ£¬
¹Ź“š°øĪŖ£ŗNa2SO4£»
£Ø4£©µāĖ®ÓŠŃõ»ÆŠŌ£¬ÄÜøÆŹ“Ļš½ŗ¹Ü£¬ĖłŅŌµāĖ®Ó¦·ÅŌŚĖįŹ½µĪ¶Ø¹ÜÖŠ½ųŠŠµĪ¶Ø£¬øł¾ŻĢāÖŠ±ķÖŠµÄŹż¾ŻæÉÖŖ£¬µŚ¶ž“ĪŹż¾ŻĘ«²ī½Ļ“ó£¬ĖłŅŌČ”Ņ»”¢ČżĮ½“ĪŹµŃéµÄŹż¾Ż£¬ĖłŅŌÓĆČ„µÄµāĖ®µÄĢå»żĪŖ$\frac{30.78+30.82}{2}$mL=30.8mL£¬µāµÄĪļÖŹµÄĮæĪŖ£ŗ0.0500mol•L-l”Į0.0308L=0.00154mol£¬
2S2O32-+I2ØTS4O62-+2I-£¬
2 1
x 0.00154mol
½āx=0.00308mol£¬¹ŹNa2S2O3•5H2OµÄĪļÖŹµÄĮæĪŖ0.00308mol£¬ÖŹĮæĪŖ£ŗ0.00308”Į248g/mol=0.7638g£¬
Ōņ³ĘČ”7.40g²śĘ·£¬ÅäÖĘ³É250mLČÜŅŗÖŠ£¬Na2S2O3•5H2OµÄÖŹĮæĪŖ=0.7638g”Į$\frac{250}{25}$=7.638g
¹Ź²śĘ·µÄ“æ¶ČĪŖ£ŗ$\frac{7.638g}{7.40g}$”Į100%=103.2%£¬
µāµ„ÖŹÓŠĒæµÄŃõ»ÆŠŌ£¬Na2SO3¾ßÓŠ»¹ŌŠŌ£¬Na2SO3»įŗĶI2·¢Éś·“Ó¦£¬“Ó¶ųÓ°Ļģ“æ¶Č£¬
¹Ź“š°øĪŖ£ŗ103.2%£»ŗ¬ÓŠµÄNa2SO3Ņ²»įŗĶI2·¢Éś·“Ó¦£¬“Ó¶ųÓ°Ļģ“æ¶Č£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹµÄÖʱø£¬ĪŖøßĘµæ¼µć£¬²ąÖŲӌѧɜµÄ·ÖĪö”¢ŹµŃéŗĶ¼ĘĖćÄÜĮ¦µÄ漲飬ĢāÄæÉę¼°ŹŌ¼ĮµÄ×÷ÓĆ”¢ĪļÖŹµÄĶʶĻ”¢µĪ¶ØµÄ¼ĘĖć”¢·½³ĢŹ½µÄŹéŠ“µČÖŖŹ¶£¬µĪ¶ØŹµŃéµÄŹż¾Ż“¦ĄķŗĶ¼ĘĖć·½·ØŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄŃ¶Č½Ļ“ó£¬×¢Ņā¹ŲĻµŹ½µÄÓ¦ÓĆ£®


 æŚĖćŠÄĖćĖŁĖćÓ¦ÓĆĢāĻµĮŠ“š°ø
æŚĖćŠÄĖćĖŁĖćÓ¦ÓĆĢāĻµĮŠ“š°ø Ķ¬²½ĶŲÕ¹ŌĶĮĻµĮŠ“š°ø
Ķ¬²½ĶŲÕ¹ŌĶĮĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  | B£® |  | C£® |  | D£® |  |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
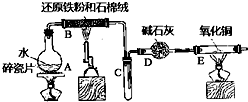 ijŹµŃ銔×éĄūÓĆĻĀĶ¼ĖłĮŠ×°ÖĆ½ųŠŠ”°ĢśÓėĖ®ÕōĘų·“Ó¦”±µÄŹµŃ飬²¢ĄūÓĆ²śĪļ½ųŅ»²½ÖĘČ”FeCl3•6H2O¾§Ģ壮£ØĶ¼ÖŠ¼Š³Ö¼°Ī²Ęų“¦Ąķ×°ÖĆ¾łŅŃĀŌČ„£©Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ijŹµŃ銔×éĄūÓĆĻĀĶ¼ĖłĮŠ×°ÖĆ½ųŠŠ”°ĢśÓėĖ®ÕōĘų·“Ó¦”±µÄŹµŃ飬²¢ĄūÓĆ²śĪļ½ųŅ»²½ÖĘČ”FeCl3•6H2O¾§Ģ壮£ØĶ¼ÖŠ¼Š³Ö¼°Ī²Ęų“¦Ąķ×°ÖĆ¾łŅŃĀŌČ„£©Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŅŅ“¼ | äåŅŅĶé | Õż¶”“¼ | 1-ä嶔Ķé | |
| ĆܶČ/£Øg•cm-3£© | 0.789 3 | 1.460 4 | 0.809 8 | 1.275 8 |
| ·Šµć/”ę | 78.5 | 38.4 | 117.2 | 101.6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
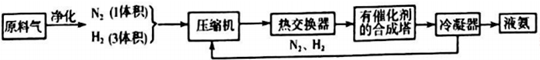
| ĘųĢå | CO | H2 | N2 | O2 |
| Ģå»ż£ØL£© | 25 | 60 | 15 | 2.5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
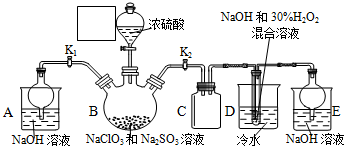
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
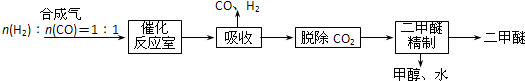
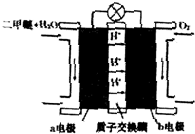
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CuSO4 | B£® | NaOH | C£® | H2SO4 | D£® | HC1 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com