
ijŠ£»Æѧъ¾æŠŌѧĻ°Š”×é¶ŌĀČĘųµÄŠŌÖŹŗĶÓĆĶ¾½ųŠŠĮĖµ÷²é·ÖĪö£¬ĮĖ½āĮĖĀČĘųÄÜÓ¦ÓĆ ÓŚŅĀĪļĘÆ°×”¢Ė®ĢåµÄɱ¾śŗĶĻū¶¾µÄŌĄķ”£
(1) ĀČĘųČÜÓŚĖ®æÉÓĆÓŚĘÆ°×”¢Ļū¶¾µÄŌŅņŹĒ_______£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_______ £»
(2) Ķس£Ź¹ÓĆĘÆ°×Ņŗ(NaClOČÜŅŗ)×÷ĘÆ°×¼Į¶ų²»ÓĆĀČĘųµÄĄķÓÉŹĒ_____(Š“³öŅ»Ļī¼“æÉ)£»
(3) ŌŚŅ»¶ØĮæµÄŹÆ»ŅČéÖŠĶØČėŅ»¶ØĮæµÄĀČĘų£¬¶žÕßĒ”ŗĆĶźČ«·“Ó¦ (·¢ÉśµÄ·“Ó¦¾łĪŖ·ÅČČ·“Ó¦£©”£×īÖÕÉś³ÉĪļÖŠŗ¬ÓŠCl-”¢ ClO-”¢ClO3-ČżÖÖŗ¬ĀČŌŖĖŲµÄĄė×Ó£¬ĘäÖŠClO-”¢ClO3-Į½ÖÖĄė ×ÓµÄĪļÖŹµÄĮæ£Øn)Óė·“Ó¦Ź±¼ä£Øt)µÄ±ä»ÆĒśĻßČēĻĀĶ¼ĖłŹ¾”£
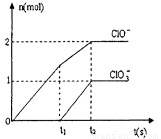
¢ŁO-t1Ź±¼äÄŚ£¬Ca(OH)2ÓėCl2·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______£»
¢Śt2Ź±£¬Ca(OH)2ÓėCl2·¢Éś·“Ó¦µÄ×ܵĻÆѧ·½³ĢŹ½ĪŖ_______
¢ŪøĆŹÆ»ŅČéÖŠŗ¬ÓŠCa(OH)2µÄÖŹĮæŹĒ_______g£»
£Ø9·Ö£©
£Ø1£©ĀČĘųŗĶĖ®·“Ӧɜ³ÉĒæŃõ»ÆŠŌµÄ“ĪĀČĖį£Ø1·Ö£©£¬ Cl2+H2O=HClO+H++ClO-£Ø1·Ö£© £»
£Ø2£©NaClOøüĪČ¶Ø£¬±ćÓŚ“¢“ęŗĶŌĖŹä£Ø1·Ö£©£»
£Ø3£©¢Ł2Ca(OH)2£«2Cl2£½Ca(C1O)2£«CaCl2£«2H2O£Ø2·Ö£©£»
¢Ś10Ca(OH)2£«10Cl2£½2Ca(C1O)2£«Ca(C1O3)2£«7CaCl2£«10H2O£Ø2·Ö£©£»
¢Ū370 g £Ø2·Ö£©
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ(1)ĀČĘųŗĶĖ®·“Ӧɜ³É“ĪĀČĖį£¬“ĪĀČĖį¾ßÓŠŗÜĒæµÄŃõ»ÆŠŌ£¬¹ŹĀČĘųČÜÓŚĖ®æÉÓĆÓŚĘÆ°×”¢Ļū¶¾µÄŌŅņŹĒĀČĘųŗĶĖ®·“Ӧɜ³ÉĒæŃõ»ÆŠŌµÄ“ĪĀČĖį£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖCl2+H2O=HClO+H++ClO-”£
(2)ĀČĘųÓŠ¶¾£¬ĒŅ²»ČŻŅ׏¹ÓĆ£¬¹ŹĶس£Ź¹ÓĆĘÆ°×Ņŗ(NaClOČÜŅŗ)×÷ĘÆ°×¼Į¶ų²»ÓĆĀČĘųµÄĄķÓÉŹĒNaClOøüĪČ¶Ø£¬±ćÓŚ“¢“ęŗĶŌĖŹä”£
(3) ¢ŁÓÉĶ¼Ļń·ÖĪö£¬t1Ź±æĢƻӊClO3-Ąė×ÓÉś³É£¬¹ŹO-t1Ź±¼äÄŚ£¬Ca(OH)2ÓėCl2·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ2Ca(OH)2£«2Cl2£½Ca(C1O)2£«CaCl2£«2H2O”£
¢ŚÓÉĶ¼Ļń·ÖĪö£¬t2Ź±ÓŠClO-”¢ClO3-Ąė×ÓÉś³É£¬¹Źt2Ź±£¬Ca(OH)2ÓėCl2·¢Éś·“Ó¦µÄ×ܵĻÆѧ·½³ĢŹ½ĪŖ10Ca(OH)2£«10Cl2£½2Ca(C1O)2£«Ca(C1O3)2£«7CaCl2£«10H2O”£
¢ŪÓÉ»Æѧ·½³Ģ¼ĘĖćµĆøĆŹÆ»ŅČéÖŠŗ¬ÓŠCa(OH)2µÄÖŹĮæŹĒ370 g”£
æ¼µć£ŗĀČĘųµÄŠŌÖŹ
µćĘĄ£ŗ±¾Ģāæ¼²éµÄŹĒĀČĘųµÄŠŌÖŹµÄĻą¹ŲÖŖŹ¶£¬ĢāÄæÄѶČÖŠµČ£¬æ¼²éѧɜ¶Ō»ł“”ÖŖŹ¶µÄÕĘĪÕ³Ģ¶ČŗĶ¼ĘĖćÄÜĮ¦”£


 ŗ®¼ŁĄÖŌ°±±¾©½ĢÓż³ö°ęÉēĻµĮŠ“š°ø
ŗ®¼ŁĄÖŌ°±±¾©½ĢÓż³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
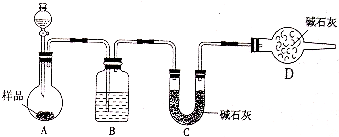
 ijŠ£»Æѧъ¾æŠŌѧĻ°Š”×éÉč¼ĘČēĻĀŹµŃé·½°ø£¬²ā¶Ø·ÅÖĆ¼ŗ¾ĆµÄŠ”ĖÕ“ņѳʷ֊“æ¼īµÄÖŹĮæ·ÖŹż£®
ijŠ£»Æѧъ¾æŠŌѧĻ°Š”×éÉč¼ĘČēĻĀŹµŃé·½°ø£¬²ā¶Ø·ÅÖĆ¼ŗ¾ĆµÄŠ”ĖÕ“ņѳʷ֊“æ¼īµÄÖŹĮæ·ÖŹż£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗŚĮś½¹ž¶ū±õŹŠµŚĮłÖŠŃ§øßČżÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©Ä³Š£»Æѧъ¾æŠŌѧĻ°Š”×éÉč¼ĘČēĻĀŹµŃé·½°ø£¬²ā¶Ø·ÅÖĆŅŃ¾ĆµÄŠ”ĖÕ“ņѳʷ֊“æ¼īµÄÖŹĮæ·ÖŹż”£
£Ø1£©·½°øŅ»£ŗ³ĘČ”Ņ»¶ØÖŹĮæµÄѳʷ£¬ÖĆÓŚŪįŪöÖŠ¼ÓČČÖĮŗćÖŲŗó£¬ĄäČ“£¬³ĘĮæŹ£Óą¹ĢĢåÖŹĮ棬¼ĘĖć”£ŹµŃéÖŠ¼ÓČČÖĮŗćÖŲµÄÄæµÄŹĒ ”£
£Ø2£©·½°ø¶ž£ŗ³ĘČ”Ņ»¶ØĮæѳʷ£¬ÖĆÓŚŠ”ÉÕ±ÖŠ£¬¼ÓŹŹĮæĖ®Čܽā£¬ĻņŠ”ÉÕ±ÖŠ¼ÓČė×ćĮæĀČ»Æ±µČÜŅŗ£¬¹żĀĖĻ“µÓ£¬øÉŌļ³Įµķ£¬³ĘĮæ¹ĢĢåÖŹĮ棬¼ĘĖć£ŗ
¢Ł¹żĀĖ²Ł×÷ÖŠ£¬³żĮĖÉÕ±”¢Ā©¶·Ķā»¹ÓƵ½µÄ²£Į§ŅĒĘ÷ÓŠ______________________£»
¢ŚŹŌŃéÖŠÅŠ¶Ļ³ĮµķŹĒ·ńĶźČ«µÄ·½·ØŹĒ_______________________________________
¢ŪČō¼ÓČėŹŌ¼ĮøÄĪŖĒāŃõ»Æ±µ£¬ŅŃÖŖ³ĘµĆѳʷ9.5g£¬øÉŌļµÄ³ĮµķÖŹĮæĪŖ19.7g£¬Ōņѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹżĪŖ_________________£Ø±£ĮōŅ»Ī»Š”Źż£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģŗŚĮś½¹ž¶ū±õŹŠøßČżÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©Ä³Š£»Æѧъ¾æŠŌѧĻ°Š”×éÉč¼ĘČēĻĀŹµŃé·½°ø£¬²ā¶Ø·ÅÖĆŅŃ¾ĆµÄŠ”ĖÕ“ņѳʷ֊“æ¼īµÄÖŹĮæ·ÖŹż”£
£Ø1£©·½°øŅ»£ŗ³ĘČ”Ņ»¶ØÖŹĮæµÄѳʷ£¬ÖĆÓŚŪįŪöÖŠ¼ÓČČÖĮŗćÖŲŗó£¬ĄäČ“£¬³ĘĮæŹ£Óą¹ĢĢåÖŹĮ棬¼ĘĖć”£ŹµŃéÖŠ¼ÓČČÖĮŗćÖŲµÄÄæµÄŹĒ ”£
£Ø2£©·½°ø¶ž£ŗ³ĘČ”Ņ»¶ØĮæѳʷ£¬ÖĆÓŚŠ”ÉÕ±ÖŠ£¬¼ÓŹŹĮæĖ®Čܽā£¬ĻņŠ”ÉÕ±ÖŠ¼ÓČė×ćĮæĀČ»Æ±µČÜŅŗ£¬¹żĀĖĻ“µÓ£¬øÉŌļ³Įµķ£¬³ĘĮæ¹ĢĢåÖŹĮ棬¼ĘĖć£ŗ
¢Ł¹żĀĖ²Ł×÷ÖŠ£¬³żĮĖÉÕ±”¢Ā©¶·Ķā»¹ÓƵ½µÄ²£Į§ŅĒĘ÷ÓŠ______________________£»
¢ŚŹŌŃéÖŠÅŠ¶Ļ³ĮµķŹĒ·ńĶźČ«µÄ·½·ØŹĒ_______________________________________
¢ŪČō¼ÓČėŹŌ¼ĮøÄĪŖĒāŃõ»Æ±µ£¬ŅŃÖŖ³ĘµĆѳʷ9.5g£¬øÉŌļµÄ³ĮµķÖŹĮæĪŖ19.7g£¬Ōņѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹżĪŖ_________________£Ø±£ĮōŅ»Ī»Š”Źż£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com