
����������Ӧ���Ȼ�ѧ����ʽ��
��I2(g) + H2(g)  ��2HI(g)��������H =��9.48 kJ��mol��1��
��2HI(g)��������H =��9.48 kJ��mol��1��
��I2(s) + H2(g)  ��2HI(g)���� �� ��H =" +26.48" kJ��mol��1
��2HI(g)���� �� ��H =" +26.48" kJ��mol��1
����˵����ȷ���ǣ� ��
| A���ٵIJ���ȢڵIJ����ȶ� |
| B��I2(s) = I2(g)��������H=" +17.00" kJ��mol��1 |
| C���ڵķ�Ӧ���������Ȣٵķ�Ӧ���������� |
| D��1mol I2(g)��ͨ��1 mol H2(g)��������Ӧʱ����9.48 kJ |
C
��������������ٵIJ���͢ڵIJ��ﶼ��HI���ȶ�����ͬ��A����ȷ���ؼ���˹���ɿ�֪���ڣ��ټ��õ�I2(s)��I2(g)����H��26.48kJ/mol��9.48kJ/mol����35.96kJ/mol��ѡ��B����ȷ������������������̬�ĵ����������Ԣڵķ�Ӧ���������Ȣٵķ�Ӧ���������ͣ�C��ȷ���÷�Ӧ�ǿ��淴Ӧ����1mol I2(g)��ͨ��1 mol H2(g)��������Ӧʱ����һ��С��9.48kJ��D����ȷ����ѡC��
���㣺���鷴Ӧ�ȵļ���ͷ�Ӧ�ȵ��й�Ӧ��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣��Ҫ�ǿ���ѧ���Է�Ӧ�Ⱥ����Լ����ø�˹���ɼ��㷴Ӧ�ȵ��˽����������ּ�ڹ���ѧ���Ļ��������ѧ����Ӧ��������ѧϰЧ�ʡ�


 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��Դ�ǵ�����ᷢչ������֧��֮һ����ר�����������ܹ�����̫����ʹȼ��ȼ�ղ����CO2��H2O��N2��������ϣ���ͼ�������Խ�Լȼ�ϣ�������ԴΣ�����ڴ˹��������ѭ����̫��������ת��Ϊ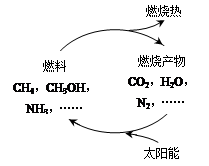
| A����ѧ�� | B������ |
| C�������� | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵���У���ȷ���� �� ��
| A�������£�pH=5.6��NaHSO3��Һ��c( SO32-)-c( H2SO3)= 10-5.6-10-8.4 |
| B��ͬ��ͬѹ�£���ӦH2(g)��Cl2(g)��2HCl(g)�ڹ��պ͵�ȼ�����µĦ�H��ͬ |
| C�����ȷ�Ӧֻ���ڼ��������²��ܷ�������ˮ�����Ȳ����� |
| D�������İ�ˮ�����ᷴӦ������Һ������Ϊ���ԣ�������Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ѧ��ӦH2��Cl2===2HCl�������仯��ͼ��ʾ��������˵����ȷ����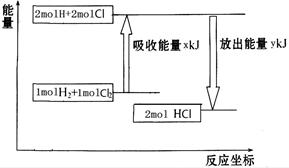
| A���÷�Ӧ�Ƿ��ȷ�Ӧ |
| B������1 mol H��H����1 mol Cl��Cl ���ų�x kJ���� |
| C������1 mol H��Cl����Ҫ����y kJ������ |
| D��2 mol HCl������������1 mol H2��1 molCl2�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵���в���ȷ����
| A����ú�͡�������Ƚϣ���Ȼ����һ�ֱȽ����Ļ�ʯȼ�� |
| B���Ҵ���һ�ֿ�������Դ����Ϊȼ�ϵ��ŵ������ȫȼ�յIJ��ﲻ��Ⱦ���� |
| C������̫���ܡ����ܺ����ܵ���Դ�����ʯ��Դ����Ч���ƿ������� |
| D��ú��������Һ���������Ի�ýྻȼ�ϣ�������ȼ�ջ�ų���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ������������⣺
��1����25�桢101kPa�£�1g�״���Һ�壬����ʽΪCH3OH��ȼ������CO2��Һ̬ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ____________________�����÷�Ӧ��Ƴɼ���ȼ�ϵ�أ�д���õ�صĸ����缫��Ӧ����ʽ ��
��2��������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㡣
��֪�� CH4(g)��H2O(g)��CO(g)��3H2(g) ��H��+206.2kJ��mol��1
CH4(g)��CO2(g)��2CO(g)��2H2(g) ��H��-247.4 kJ��mol��1
�Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ����CH4(g)��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽΪ ��
��3����֪���ס�����ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ
P4(s,����)+ 5O2=P4O10(s)����H���C2986kJ��mol��1
4P(s,����)+ 5O2=P4O10(s)����H���C2956kJ��mol��1
����ױȺ��� ����ȶ������ȶ�����
��4�� ��֪һ��������A2��B2�Է���Ӧ����AB3����Ӧ
A2(g)+3B2(g)=2AB3(g) �Ħ�S= 0����H 0 ���<������>������=����
��5����ͼΪ��⾫������ʾ��ͼ�� ����a��b����Ϊ�������ʵĴ� ������b������������ɫ�������ɣ������ɸ�����ĵ缫��ӦʽΪ 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4�֣���ҵ�ϳ�����Ȼ����Ϊ�Ʊ��״�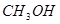 ��ԭ�ϡ���֪��
��ԭ�ϡ���֪��
��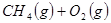
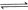
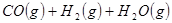
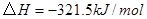
��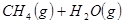
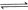
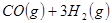
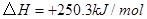
��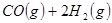
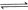
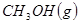
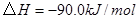
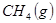 ��
��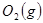 ��Ӧ����
��Ӧ����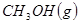 ���Ȼ�ѧ����ʽΪ ��
���Ȼ�ѧ����ʽΪ ��
��2����VL�����ܱ������г���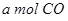 ��
��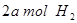 ���ڲ�ͬѹǿ�ºϳɼ״���
���ڲ�ͬѹǿ�ºϳɼ״��� ��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��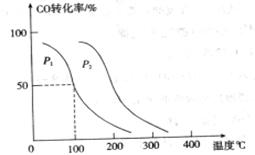
��ѹǿ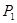
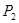 ���<������>����=����
���<������>����=����
����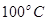 ��
��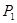 ѹǿʱ��ƽ�ⳣ��Ϊ ���ú�
ѹǿʱ��ƽ�ⳣ��Ϊ ���ú�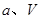 �Ĵ���ʽ��ʾ����
�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CO�dz����Ļ�ѧ���ʣ��ڹ�ҵ��������;�ܹ㷺��
��1�� ��֪��ijЩ��Ӧ���Ȼ�ѧ����ʽ���£�
2H2��g��+SO2��g��=S��g��+2H2O��g�� ��H��+90.4kJ��mol��1
2CO��g��+O2��g��=2CO2��g�� ��H��-556.0kJ��mol��1
2H2��g��+O2��g��=2H2O��g�� ��H��-483.6kJ��mol��1
��д����CO��ȥ������SO2������S��g����CO2�Ȼ�ѧ����ʽ
��2�� ijȼ�ϵ����COΪȼ�ϣ��Կ���Ϊ��������������̬��K2CO3Ϊ����ʣ���д����ȼ�ϵ�������ĵ缫��Ӧʽ ��
��3����ij�¶��¡��ݻ���Ϊ2L�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��º��ݣ�ʹ֮������Ӧ��2H2��g����CO��g�� CH3OH��g������H����dJ��mol��1��d��0������ʼͶ����������ﵽƽ��ʱ���й��������£�
CH3OH��g������H����dJ��mol��1��d��0������ʼͶ����������ﵽƽ��ʱ���й��������£�
| ʵ�� | �� | �� | �� |
| ��ʼͶ�� | 2 molH2��1 molCO | 1 mol CH3OH | 4 molH2��2 molCO |
| ƽ��ʱn��CH3OH �� | 0.5mol | n2 | n3 |
| ��Ӧ�������仯 | �ų�Q1kJ | ����Q2kJ | �ų�Q3kJ |
| ��ϵ��ѹǿ | P1 | P2 | P3 |
| ��Ӧ���ת���� | ��1 | ��2 | ��3 |
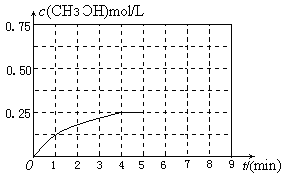
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com