
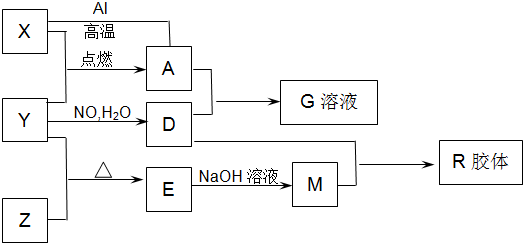
ͼ��X��Y��ZΪ���ʣ�����Ϊ���������֮���������ת����ϵ�����ֲ�������ȥ�������У�A�׳ƴ�����������E�Dz�����ˮ�������������������ᷴӦ��
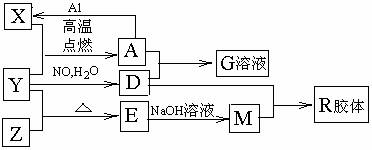
�ش��������⣺
����ɵ���Y��Ԫ�������ڱ��е�λ���� ��M�д��ڵĻ�ѧ������Ϊ ��R�Ļ�ѧʽ�� ��
��һ�������£�Z��H2��Ӧת��ΪZH4��ZH4�ĵ���ʽΪ ��
 ����֪A��1mol Al��Ӧת��ΪXʱ���������ʾ�Ϊ���壩���ų�akJ������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
����֪A��1mol Al��Ӧת��ΪXʱ���������ʾ�Ϊ���壩���ų�akJ������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��д��A��D��ϡ��Һ��Ӧ����G�����ӷ���ʽ�� ��
����4mol D��ϡ��Һ�У�����X��ĩ���������������ɵ�����ֻ��һ�֣���������ϵ�л���n(X2+)��n(X)�仯��ʾ��ͼ�������n(X2+)�����ֵ��
�ŵڶ����ڵ�VIA�� ���Ӽ������ۼ� H2SiO3(��H4SiO4) ��![]()
��8Al(s)+3Fe3O4(s)=9Fe(s)+4Al2O3(s) ��H=-8a kJ/mol
��3Fe3O4+28H��+NO3��![]() 9Fe3��+NO��+14H2O ��
9Fe3��+NO��+14H2O ��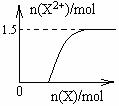
�����������������Ļ�ѧ�ɷ���Fe3O4����AΪFe3O4���ݴ˼�������ȷ��XΪ����YΪO2��DΪHNO3����������ᷴӦ�Ҳ�����ˮ��������������SiO2����EΪSiO2����ZΪSi��MΪNa2SiO3��RΪH2SiO3��H4SiO4�����������ƶϼ��ɻش��й����������ˣ�������𰸡�


 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


 9Fe3++NO��+14H2O
9Fe3++NO��+14H2O 9Fe3++NO��+14H2O
9Fe3++NO��+14H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��Ϫһ�и߶��ڶ����¿���ѧ�Ծ����������� ���ͣ������
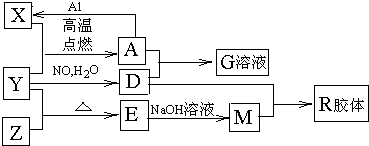
(16��)ͼ��X��Y��ZΪ���ʣ�����Ϊ��ѧ�����֮���������ת����ϵ�����ֲ�������ȥ�������У�A�׳ƴ�����������E�Dz�����ˮ�������������������ᷴӦ��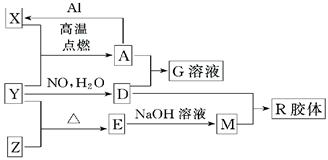
�ش��������⣺
����ɵ���Y��Ԫ�������ڱ��е�λ���� ��M�д��ڵĻ�ѧ������ Ϊ ��R�Ļ�ѧʽ�� ��
��һ�������£�Z��H2��Ӧ����ZH4,ZH4�ĵ���ʽΪ ��
����֪A��1molAl��Ӧת��ΪXʱ���������ʾ�Ϊ���壩���ų�a KJ������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��д��A��D��ϡ��Һ��Ӧ����G�����ӷ���ʽ�� ��ת��0.6mol���ӣ����ɵ������ڱ�������Ϊ
����4mol D��ϡ��Һ�У�����X��ĩ���������������ɵ�����ֻ��һ�֣���������ϵ�л���n(X2+)��n��X���仯��ʾ��ͼ�������n(X2+)�����ֵ��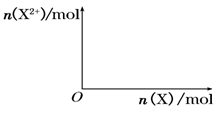
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ������ѧ�ڵڶ��ζ�ʱ��ϰ��ѧ�Ծ� ���ͣ������
��7�֣�ͼ��X��Y��ZΪ���ʣ�����Ϊ���������֮���������ת����ϵ�����ֲ�������ȥ�������У�A�׳ƴ�����������E�Dz�����ˮ�������������������ᷴӦ���ش��������⣺
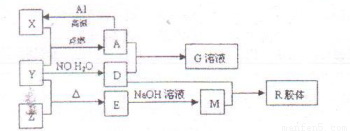
��1����ɵ���Y��Ԫ�������ڱ��е�λ���� ��M�д��ڵĻ�ѧ������Ϊ ��R�Ļ�ѧʽ�� ��
��2����֪A��1mol Al��Ӧת��ΪXʱ���������ʾ�Ϊ���壩���ų�akJ���� ��д���÷�Ӧ���Ȼ�ѧ����ʽ��
��3��д��A��D��ϡ��Һ��Ӧ����G�����ӷ���ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com