
ij¹¤³§ÅųöµÄĪŪĖ®ÖŠŗ¬ÓŠ“óĮæµÄFe2£«”¢Zn2£«”¢Hg2£«ČżÖÖ½šŹōĄė×Ó”£ŅŌĻĀŹĒij»Æѧъ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§Éč¼ĘµÄ³żČ„ĪŪĖ®ÖŠµÄ½šŹōĄė×Ó£¬»ŲŹÕĀĢ·Æ”¢š©·Æ(ZnSO4·7H2O)ŗĶ¹ÆµÄ·½°ø”£
[Ņ©Ę·]””NaOHČÜŅŗ”¢Įņ»ÆÄĘČÜŅŗ”¢Įņ»ÆŃĒĢś”¢Ļ”ĮņĖį”¢Ģś·Ū
[ŹµŃé·½°ø]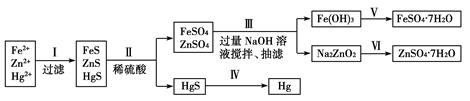
[ĪŹĢāĢ½¾æ]
£Ø1£©²½Öč¢ņĖł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_________________________________________________”£
£Ø2£©²½Öč¢óÖŠµÄ³éĀĖĪŖ¼õŃ¹Ģõ¼žĻĀµÄ¹żĀĖ£¬æÉŅŌ¼Óæģ¹żĀĖĖŁ¶Č£»øĆ²½ÖčÉę¼°·“Ó¦µÄĄė×Ó·½³ĢŹ½ÓŠZn2£«£«4OH£=ZnO22-£«2H2OŗĶ________________”£
£Ø3£©²½Öč¢öÖŠµĆµ½ĮņĖįŠæČÜŅŗµÄĄė×Ó·“Ó¦·½³ĢŹ½ĪŖ______________________________”£
£Ø4£©ÓūŹµĻÖ²½Öč¢õ£¬ĖłŠč¼ÓČėµÄŹŌ¼ĮÓŠ________”¢________£¬ĖłÉę¼°µÄÖ÷ŅŖ²Ł×÷ŅĄ“ĪĪŖ______________________”£
£Ø5£©²½Öč¢ō³£ÓƵķ½·ØŹĒ¼ÓČČ£¬øĆ²½ÖčŹĒ·ń¶Ō»·¾³ÓŠÓ°Ļģ£æ__________(Ģī”°ŹĒ”±»ņ”°·ń”±)£¬ČēÓŠÓ°Ļģ£¬ĒėÄćÉč¼ĘŅ»øö»·¾³±£»¤·½°øĄ“ŹµĻÖ²½Öč¢ōµÄ·“Ó¦________________________”£
£Ø1£©FeS£«2H£«=Fe2£«£«H2S”ü£¬ZnS£«2H£«=Zn2£«£«H2S”ü
£Ø2£©4Fe2£«£«O2£«8OH££«2H2O=4Fe(OH)3”ż
£Ø3£©ZnO22-£«4H£«=Zn2£«£«2H2O
£Ø4£©Ļ”ĮņĖį””Ģś·Ū””¹żĀĖ”¢ÅØĖõ½į¾§”¢¹żĀĖ
£Ø5£©””ŹĒ””ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČČHgS
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©²½Öč¢ņ¼ÓČėĻ”ĮņĖį£¬ÓėFeS”¢ZnS·Ö±š·¢Éśø“·Ö½ā·“Ó¦£¬æÉŠ“³ÉĄė×Ó·½³ĢŹ½”£
£Ø2£©FeSO4”¢ZnSO4»ģŗĻČÜŅŗÖŠ¼ÓČė¹żĮæµÄNaOHŹ±£¬Fe2£«ÓėOH£·“Ӧɜ³ÉFe(OH)2£¬Fe(OH)2ŃøĖŁ±»æÕĘųÖŠµÄO2Ńõ»ÆĪŖFe(OH)3£¬Ęä×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ4Fe2£«£«O2£«8OH££«2H2O=4Fe(OH)3”ż”£
£Ø3£©øł¾ŻæņĶ¼×Ŗ»Æ¹ŲĻµ£¬æÉŅŌ擳öZn(OH)2µÄŠŌÖŹĄąĖĘÓŚAl(OH)3£¬ĖłŅŌNa2ZnO2ČÜŅŗÖŠ¼ÓČė¹żĮæµÄH2SO4Éś³ÉZnSO4ČÜŅŗ£¬ĘäĄė×Ó·½³ĢŹ½ĪŖ£ŗZnO22-£«4H£«=Zn2£«£«2H2O”£
£Ø4£©ĒāŃõ»ÆĢśÄÜÓėĻ”ĮņĖį·“Ӧɜ³ÉĮņĖįĢś£¬ĮņĖįĢśÄÜÓė¹żĮæĢśµ„ÖŹ·“Ӧɜ³É¶ž¼ŪĢśĄė×Ó£¬Č»ŗó¹żĀĖ”¢ÅØĖõ½į¾§”¢¹żĀĖµĆµ½FeSO4?7H2O”£
£Ø5£©HgSŌŚæÕĘųÖŠ¼ÓČČæɵĆHg”¢SO2£¬HgÕōĘųŗĶSO2¶¼»į¶Ō»·¾³²śÉśĪŪČ¾£¬µ«ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČČHgSæÉÓŠŠ§·ĄÖ¹HgÕōĘųŗĶSO2ŹĶ·Åµ½“óĘųÖŠ£¬“Ó¶ų±£»¤ĮĖ»·¾³”£
æ¼µć£ŗ±¾Ģāæ¼²é»Æѧ¹¤ŅÕĮ÷³ĢµÄ·ÖĪö£¬·½³ĢŹ½µÄŹéŠ“”¢ŹŌ¼ĮµÄŃ”ŌńŗĶ»ł±¾²Ł×÷”¢»Æѧ·½°øµÄ·ÖĪö”£


 Ó¦ÓĆĢāµć²¦ĻµĮŠ“š°ø
Ó¦ÓĆĢāµć²¦ĻµĮŠ“š°ø דŌŖ¼°µŚĻµĮŠ“š°ø
דŌŖ¼°µŚĻµĮŠ“š°ø Ķ¬²½°ĀŹżĻµĮŠ“š°ø
Ķ¬²½°ĀŹżĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŗ£ŃóŹĒÉśĆüµÄŅ”Ąŗ”¢×ŹŌ“µÄ±¦æā”£ÖŠ¹śŅŖŹµŹ©ŗ£ŃóĒæ¹śÕ½ĀŌ£¬ŹµĻÖÓÉŗ£Ńó“ó¹śĻņŗ£ŃóĒæ¹śĀõ½ųµÄĆĪĻė”£ŗ£Ńó¾¼ĆŅŃ¾³ÉĪŖĄ¶ÆĪŅ¹ś¹śĆń¾¼Ć·¢Õ¹µÄÖŲŅŖŅżĒę£¬ŗ£Ė®µÄ×ŪŗĻæŖ·¢”¢ĄūÓĆŹĒŗ£Ńó¾¼ĆµÄŅ»²æ·Ö£¬ŗ£Ė®ÖŠæÉĢįČ”¶ąÖֻƹ¤ŌĮĻ£¬ĻĀĆęŹĒ¹¤ŅµÉĻ¶Ōŗ£Ė®µÄ¼øĻī×ŪŗĻĄūÓƵď¾ŅāĶ¼”£ĘäĮ÷³ĢČēĻĀĶ¼ĖłŹ¾£ŗ
£Ø1£©Š“³ö¢Ł”¢¢Ś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
¢Ł______________________£¬¢Ś______________________”£
£Ø2£©¹¤ŅµÉĻĄūÓƵē½ā±„ŗĶŹ³ŃĪĖ®²śÉśµÄĒāĘųŗĶĀČĘųÖĘČ”ŃĪĖį£¬ĪŖĮĖĢåĻÖĀĢÉ«»ÆѧĄķÄī£¬Ź¹ĀČĘų³ä·Ö·“Ó¦£¬²ÉČ”½«ĀČĘųŌŚĒāĘųÖŠČ¼Éյİģ·Ø£¬æɱÜĆāĀČĘųČ¼ÉÕ²»ĶźČ«ĪŪČ¾æÕĘų£¬ĒėŠ“³öĀČĘųŌŚĒāĘųÖŠČ¼ÉÕµÄŹµŃéĻÖĻó£ŗ______________________”£
£Ø3£©“ÖŃĪÖŠŗ¬ÓŠCa£²£«”¢Mg£²£«”¢SO4£²£µČŌÓÖŹ£¬¾«ÖĘŗóæɵƵ½±„ŗĶNaC1ČÜŅŗ”£ĻÖÓŠĻĀĮŠ³żŌÓŹŌ¼Į£ŗA£®ŃĪĖį B£®ĒāŃõ»Æ±µČÜŅŗ C£®Ģ¼ĖįÄĘČÜŅŗ”£¾«ÖĘŹ±¼ÓČė¹żĮæ³żŌÓŹŌ¼ĮµÄÕżČ·Ė³ŠņŹĒ______________”££ØĢīŠņŗÅ£©
£Ø4£©½šŹōĆ¾ŌŚæÕĘųÖŠČ¼ÉÕŹ±£¬³żÉś³ÉMgOĶā£¬»¹ÓŠÉŁĮæMg3N2Éś³É”£°ŃµČĪļÖŹµÄĮæµÄ½šŹōĆ¾·Ö±š·ÅŌŚ£ŗA£®“æŃõĘų£ØO2£©ÖŠ£»B£®¶žŃõ»ÆĢ¼ĘųĢåÖŠ£»C£®æÕĘųÖŠ”£ĶźČ«Č¼ÉÕŗó£¬µĆµ½µÄ¹ĢĢåĪļÖŹµÄÖŹĮæÓɓ󵽊”µÄĖ³ŠņŹĒ______________”££ØĢīŠņŗÅ£©
£Ø5£©½«µē½ā±„ŗĶNaClČÜŅŗÉś³ÉµÄĀČĘųĶØČėĒāŃõ»ÆÄĘČÜŅŗÖŠæÉŅŌµĆµ½NaClO”£Ä³»ÆѧŠĖȤŠ”×éĢ½¾æNaClOÓėÄņĖŲCO(NH2)2µÄ·“Ó¦²śĪļ£¬ĶعżŹµŃé·¢ĻÖ²śĪļ³żÄ³ÖÖŃĪĶā£¬ĘäÓą²śĪļ¶¼ŹĒÄܲĪÓė“óĘųŃ»·µÄĪļÖŹ£¬ŌņøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŗ£Ė®ÖŠÓŠ·įø»µÄ׏Ō“£¬¶ąÖÖ¶ąŃłµÄŗ£Ńó¶ÆĪļŗĶÖ²Īļ£¬ŗ£µ×ÓŠ·įø»µÄæó²Ų”¢ŹÆÓĶ”¢ĢģČ»ĘųµČ£¬“ĖĶā£¬ŗ£Ė®ÖŠ»¹ŗ¬ÓŠ“óĮæµÄµē½āÖŹ£¬ĖüĆĒµēĄė²śÉśCl£”¢Br££ØäåĄė×Ó£©”¢SO42£”¢Na£«”¢Mg2£«”¢Ca2£«µČ£¬¶¼ŹĒÖŲŅŖ׏Ō“”£
£Ø1£©Š“³ö²½Öč¢Ł”¢¢Ś”¢¢Ü·ÖĄėĢį“æµÄ·½·Ø£ŗ
¢Ł £» ¢Ś £» ¢Ü ”£
£Ø2£©²½Öč¢Ś·ÖĄėĢį“æ¹ż³ĢÖŠŠčŅŖŃ”ÓĆÖ÷ŅŖ²£Į§ŅĒĘ÷µÄĆū³Ę ”£
£Ø3£©Óū³żČ„³õ²½Ģį“æŗóµÄ“ÖŃĪÖŠµÄMgCl2”¢CaCl2ŗĶNa2SO4£¬Ó¦ĻņøĆ“ÖŹ³ŃĪĖ®ÖŠŅĄ“Ī¼ÓČėNaOHČÜŅŗ”¢ ČÜŅŗ”¢ ČÜŅŗ£¬Č»ŗó¹żĀĖ£»ĪŖ¾”æÉÄܳżČ„ŌÓÖŹ£¬Ćæ“Ī¼ÓČėµÄŹŌ¼ĮÓ¦ ”£ĻņĖłµĆČÜŅŗÖŠµĪ¼Ó ÖĮĪŽĘųÅŻ²śÉś£¬ŌŁ¾Õō·¢½į¾§µĆµ½Ź³ŃĪ¾§Ģ唣
£Ø4£©¼ģŃéµĖ®ÖŠŹĒ·ńŗ¬ÓŠCl-µÄ²Ł×÷ĻÖĻó¼°½įĀŪ ”£
£Ø5£©Š“³ö¼ÓČėŹŌ¼Įaŗ󣬷¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
£Ø6£©¹¤ŅµÉĻÓƵē½ā±„ŗĶŹ³ŃĪĖ®µÄ·½·ØÉś²śĀČĘų”¢ĒāĘųŗĶÉÕ¼ī£¬ĒėŠ“³öĄūÓĆĀČĘųŗĶŹÆ»ŅČéÖĘČ”ĘÆ°×·ŪµÄ»Æѧ·½³ĢŹ½ ________________________________________________ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŌ»ĘĢśæóĪŖŌĮĻÖĘĮņĖį²śÉśµÄĮņĖįŌüÖŠŗ¬Fe2O3”¢SiO2”¢Al2O3”¢MgOµČ”£ŹµŃéŹŅÄ£Äā¹¤ŅµŅŌĮņĖįŌüÖʱøĢśŗģ(Fe2O3)£¬¹ż³ĢČēĻĀ£ŗ
£Ø1£©ĮņĖįŌüµÄ³É·ÖÖŠŹōÓŚĮ½ŠŌŃõ»ÆĪļµÄŹĒ £¬ Š“³öĖįČܹż³ĢFe2O3ÓėĻ”ĮņĖį·“Ó¦µÄĄė×Ó·“Ó¦·½³ĢŹ½£ŗ £»
£Ø2£©Éś²ś¹ż³ĢÖŠ£¬ĪŖĮĖČ·±£ĢśŗģµÄ“æ¶Č£¬Ńõ»Æ¹ż³ĢŠčŅŖµ÷½ŚČÜŅŗµÄpHµÄ·¶Ī§ŹĒ_________£»£Ø²æ·ÖŃōĄė×ÓŅŌĒāŃõ»ÆĪļŠĪŹ½³ĮµķŹ±ČÜŅŗµÄpH¼ūĻĀ±ķ£©
| ³ĮµķĪļ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 |
| æŖŹ¼³Įµķ | 2.7 | 3.8 | 7.5 | 9.4 |
| ĶźČ«³Įµķ | 3.2 | 5.2 | 9.7 | 12.4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
øõĢśæóµÄÖ÷ŅŖ³É·ÖæɱķŹ¾ĪŖFeO”¤Cr2O3£¬»¹ŗ¬ÓŠMgO”¢Al2O3”¢Fe2O3µČŌÓÖŹ£¬ŅŌĻĀŹĒŅŌøõĢśæóĪŖŌĮĻÖʱøÖŲøõĖį¼Ų£ØK2Cr2O7£©µÄĮ÷³ĢĶ¼£ŗ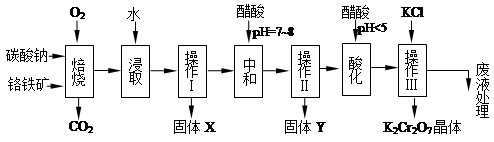
ŅŃÖŖ£ŗ¢Ł4FeO”¤Cr2O3+ 8Na2CO3+ 7O2 8Na2CrO4 + 2 Fe2O3 + 8CO2”ü£»
8Na2CrO4 + 2 Fe2O3 + 8CO2”ü£»
¢ŚNa2CO3 + Al2O3 2NaAlO2 + CO2”ü£»¢Ū Cr2O72£+ H2O
2NaAlO2 + CO2”ü£»¢Ū Cr2O72£+ H2O 2CrO42£ + 2H+
2CrO42£ + 2H+
øł¾ŻĢāŅā»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¹ĢĢåXÖŠÖ÷ŅŖŗ¬ÓŠ_________£ØĢīŠ“»ÆѧŹ½£©£»ŅŖ¼ģ²āĖį»Æ²Ł×÷ÖŠČÜŅŗµÄpHŹĒ·ńµČÓŚ4.5£¬Ó¦øĆŹ¹ÓĆ__________£ØĢīŠ“ŅĒĘ÷»ņŹŌ¼ĮĆū³Ę£©”£
£Ø2£©Ėį»Æ²½ÖčÓĆ“×Ėįµ÷½ŚČÜŅŗpH<5£¬ĘäÄæµÄŹĒ_________________________________”£
£Ø3£©²Ł×÷¢óÓŠ¶ą²½×é³É£¬»ńµĆK2Cr2O7¾§ĢåµÄ²Ł×÷ŅĄ“ĪŹĒ£ŗ¼ÓČėKCl¹ĢĢ唢Õō·¢ÅØĖõ”¢ ”¢¹żĀĖ”¢_______”¢øÉŌļ”£
£Ø4£©ĻĀ±ķŹĒĻą¹ŲĪļÖŹµÄČܽā¶ČŹż¾Ż£¬²Ł×÷¢ó·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ£ŗNa2Cr2O7+2KCl ”śK2Cr2O7”ż+2NaCl”£øĆ·“Ó¦ŌŚČÜŅŗÖŠÄÜ·¢ÉśµÄĄķÓÉŹĒ_______________”£
| ĪļÖŹ | Čܽā¶Č/(g/100gĖ®) | ||
| 0”ćC | 40”ćC | 80”ćC | |
| KCl | 28 | 40.1 | 51.3 |
| NaCl | 35.7 | 36.4 | 38 |
| K2Cr2O7 | 4.7 | 26.3 | 73 |
| Na2Cr2O7 | 163 | 215 | 376 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅųĶŗĻ½š¹ć·ŗÓ¦ÓĆÓŚŗ½æÕ¹¤Ņµ”£“ÓĒŠøī·ĻĮĻÖŠ»ŲŹÕŅų²¢ÖʱøĶµÄ»Æ¹¤²śĘ·CuAlO2µÄ¹¤ŅÕČēĻĀ£ŗ£Ø×¢£ŗAl(OH)3ŗĶCu(OH)2æŖŹ¼·Ö½āµÄĪĀ¶Č·Ö±šĪŖ450”ęŗĶ80”ę£©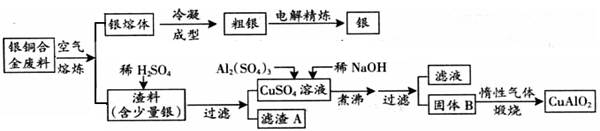
£Ø1£©ĶµÄŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ .
£Ø2£©µē½ā¾«Į¶ŅųŹ±£¬Ņõ¼«·“Ó¦Ź½ĪŖ ĀĖŌüAÓėĻ”HNO3·“Ó¦£¬²śÉśµÄĘųĢåŌŚæÕĘųÖŠŃøĖŁ±äĪŖŗģ×ŲÉ«£¬øĆĘųĢå±äÉ«µÄ»Æѧ·½³ĢŹ½ĪŖ .
£Ø3)¹ĢĢå»ģŗĻĪļBµÄ×é³ÉĪŖ £»ŌŚÉś³É¹ĢĢåBµÄ¹ż³ĢÖŠ£¬ŠčæŲÖĘNaOHµÄ¼ÓČėĮ棬ČōNaOH¹żĮ棬×īŗó½«µĆ²»µ½CuAlO2 £¬Š“³öŅņNaOH¹żĮæŅżĘšµÄ·“Ó¦µÄĄė×Ó·½³ĢŹ½ .
£Ø4£©Čō½«ÉĻŹöĮ÷³ĢĶ¼ÖŠ¼ÓČėµÄĻ”NaOHČÜŅŗøÄĪŖ¼ÓČė¹żĮæµÄ°±Ė®£ØĘäĖü¾ł²»±ä£©£¬ŌņĀĖŅŗÖŠµÄŃōĄė×ÓÓŠ .
£Ø5£©Ķź³ÉÉĻŹöģŃÉÕ¹ż³ĢÖŠŅ»øö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
CuO £« Al2O3 Cu AlO2 £« £ØĻµŹż1Ņ²ŅŖŠ“£©.
Cu AlO2 £« £ØĻµŹż1Ņ²ŅŖŠ“£©.
£Ø6£©ČōŅųĶŗĻ½šÖŠĶµÄÖŹĮæ·ÖŹżĪŖ64%£¬ĄķĀŪÉĻ5.0kg·ĻĮĻÖŠµÄĶæÉĶźČ«×Ŗ»ÆĪŖCuAlO2£¬ÖĮÉŁŠčŅŖ1.0mol·L£1µÄAl2(SO4)3ČÜŅŗ L .
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø¢ń£©¶žŃõ»Æīę£ØCeO2£©ŹĒŅ»ÖÖÖŲŅŖµÄĻ”ĶĮŃõ»ÆĪļ”£Ę½°åµēŹÓĻŌŹ¾ĘĮÉś²ś¹ż³ĢÖŠ²śÉś“óĮæµÄ·Ļ²£Į§·ŪÄ©£Øŗ¬SiO2”¢Fe2O3”¢CeO2ŅŌ¼°ĘäĖūÉŁĮææÉČÜÓŚĻ”ĖįµÄĪļÖŹ£©”£Ä³æĪĢā×éŅŌ“Ė·ŪÄ©ĪŖŌĮĻ»ŲŹÕīę£¬Éč¼ĘŹµŃéĮ÷³ĢČēĻĀ£ŗ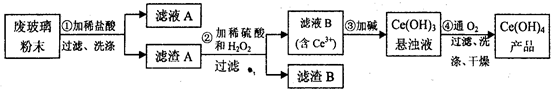
£Ø1£©Ļ“µÓµÄÄæµÄÖ÷ŅŖŹĒĪŖĮĖ³żČ„Cl£ŗĶ___________£ØĢīĄė×Ó·ūŗÅ£©£¬¼ģŃéøĆĀĖŌüAĻ“¾»µÄ·½·ØŹĒ_____________________”£
£Ø2£©µŚ¢Ś²½·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ____________________________”£
£Ø3£©Č”ÉĻŹöĮ÷³ĢÖŠµĆµ½µÄCe£ØOH£©4²śĘ·0£®536 g£¬¼ÓĮņĖįČܽāŗó£¬ÓĆ0£®1000mol· L£1FeSO4±ź×¼ČÜŅŗµĪ¶ØÖÕµćŹ±£Øīę±»»¹ŌĪŖCe3£«£©£¬ĻūŗÄ25£®00mL±ź×¼ČÜŅŗ£¬øĆ²śĘ·ÖŠCe£ØOH£©4µÄÖŹĮæ·ÖŹżĪŖ_____________”£
£Ø¢ņ£©Ńõ»ÆĆ¾ŌŚŅ½Ņ©”¢½ØÖžµČŠŠŅµÓ¦ÓĆ¹ć·ŗ£®ĮņĖįĆ¾»¹ŌČČ½āÖʱøøß“æŃõ»ÆĆ¾ŹĒŅ»ÖÖŠĀµÄĢ½Ė÷£®ŅŌĮāĆ¾æó£ØÖ÷ŅŖ³É·ÖĪŖMgCO3£¬ŗ¬ÉŁĮæFeCO3£©ĪŖŌĮĻÖʱøøß“æŃõ»ÆĆ¾µÄŹµŃéĮ÷³ĢČēĻĀ£ŗ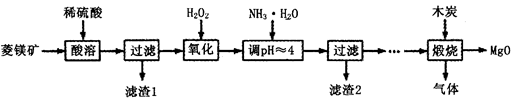
£Ø1£©¼ÓČėH2O2Ńõ»ÆŹ±£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________”£
£Ø2£©ĀĖŌü2µÄ³É·ÖŹĒ______________£ØĢī»ÆѧŹ½£©”£
£Ø3£©ģŃÉÕ¹ż³Ģ“ęŌŚŅŌĻĀ·“Ó¦£ŗ2MgSO4£«C=2MgO£«2SO2”ü£«CO2”ü
MgSO4£«C=MgO£«SO2”ü£«CO”ü MgSO4£«3C=MgO£«S”ü£«3CO”ü
ĄūÓĆČēĶ¼×°ÖƶŌģŃÉÕ²śÉśµÄĘųĢå½ųŠŠ·Ö²½ĪüŹÕ»ņŹÕ¼Æ”£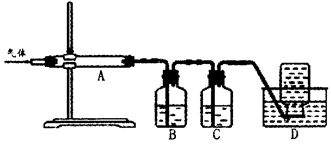
¢ŁDÖŠŹÕ¼ÆµÄĘųĢåæÉŅŌŹĒ______________£ØĢī»ÆѧŹ½£©”£
¢ŚBÖŠŹ¢·ÅµÄČÜŅŗæÉŅŌŹĒ______________£ØĢī×ÖÄø£©”£
| A£®NaOHČÜŅŗ | B£®Ca£ØOH£©2ČÜŅŗ | C£®Ļ”ĻõĖį | D£®KMnO4ČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¼ų±šĻĀĮŠø÷×éĪļÖŹ£¬°“ŅŖĒó»Ų“šĪŹĢā”£
(1)Ļ”ĮņĖį”¢Ļ”ŃĪĖį
¢ŁæÉŃ”×÷¼ų±šµÄŹŌ¼ĮÓŠ£ØĢī±ąŗÅ£¬æɶąŃ”£©_________”£
A£®BaCl2ČÜŅŗ B£®Mg(NO3)2ČÜŅŗ C£®Na2CO3ČÜŅŗ
¢Ś¼ų±š·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ_________________________”£
(2)±„ŗĶĢ¼ĖįÄĘČÜŅŗ”¢³ĪĒåŹÆ»ŅĖ®
¢ŁæÉŃ”ÓĆ¼ų±šµÄŹŌ¼ĮÓŠ£ØĢī±ąŗÅ£¬æɶąŃ”£©____”£
a£®ŃĪĖį b£®NaCI c£®ĻõĖį d£®BaCl2ČÜŅŗ
¢ŚŅĄ“ĪŠ“³ö¼ų±šÖŠÓŠĆ÷ĻŌĻÖĻóµÄ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
£Ø14·Ö£©Īų34SeŗĶķŚ52Te¶¼ŹĒµŚVIA×åŌŖĖŲ£¬ĪųŹĒ·Ö²¼ŌŚµŲæĒÖŠµÄĻ”ÓŠŌŖĖŲ”£¹¤ŅµÉĻÓĆĪų¹Ä·ĻĮĻ£ØÖ÷ŅŖ³É·ÖĪų”¢ķŚ”¢Ģ¼”¢ĶŗĶĢśŗĻ½š£©»ŲŹÕ¾«Į¶ĪųµÄĮ÷³ĢČēĻĀ£ŗ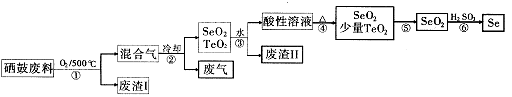
ŅŃÖŖ£ŗ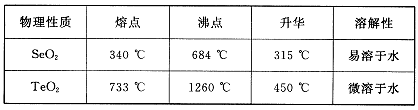
£Ø1£©SeµÄĒā»ÆĪļµÄµē×ÓŹ½ŹĒ____”£
£Ø2£©²½Öč¢ŁÖŠĶØČĖµÄŃõĘųŹ¹Īų¹Ä·ĻĮĻ·ĢŚ£¬ÄæµÄŹĒ ”£
£Ø3£©·ĻĘųµÄÖ÷ŅŖ³É·ÖŹĒ____ £»·ĻŌüIIµÄÖ÷ŅŖ³É·ÖŹĒ ”£
£Ø4£©²½Öč¢ÜÖŠÖ÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ £»²½Öč¢Ž·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø5£©øł¾Ż±ķÖŠŹż¾Ż£¬²½Öč¢Ż×īŹŹŅĖµÄ·ÖĄė·½·ØŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com