
ij½šŹō£ØA£©ŌŚTKŅŌĻĀ¾§ĢåµÄ»ł±¾½į¹¹µ„ŌŖČē×óĻĀĶ¼ĖłŹ¾£¬T KŅŌÉĻ×Ŗ±äĪŖÓŅĻĀĶ¼ĖłŹ¾½į¹¹µÄ»ł±¾½į¹¹µ„ŌŖ£¬ŌŚĮ½ÖÖ¾§ĢåÖŠ×īĮŚ½üµÄAŌ×Ó¼ä¾ąĄėĻąĶ¬
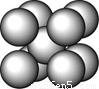

£Ø1£©ŌŚT KŅŌĻĀµÄ“æA¾§ĢåÖŠ£¬ÓėAŌ×ÓµČ¾ąĄėĒŅ×ī½üµÄAŌ×ÓŹżĪŖ______øö£»ŌŚT KŅŌÉĻµÄ“æA¾§ĢåÖŠ£¬ÓėAŌ×ÓµČ¾ąĄėĒŅ×ī½üµÄAŌ×ÓŹżĪŖ___________£»
£Ø2£©“æA¾§ĢåŌŚ¾§ŠĶ×Ŗ±äĒ°ŗ󣬶žÕß»ł±¾½į¹¹µ„ŌŖµÄ±ß³¤Ö®±ČĪŖ£ØTKŅŌÉĻÓėTKŅŌĻĀÖ®±Č£©___________”£
£Ø3£©×óÉĻĶ¼µÄµÄ¶Ń»ż·½Ź½ĪŖ £¬ ¾²ā¶ØĘä½į¹¹ŗĶŠŌÖŹ²ĪŹżČēĻĀ±ķĖłŹ¾
|
½šŹō |
Ļą¶ŌŌ×ÓÖŹĮæ |
·ÖĒų |
ĆܶČ/g”¤©M-3 |
Ō×Ó»ÆČČ/kJ”¤mol-1 |
|
Na |
22.99 |
sĒų |
0.960 |
108.4 |
|
A |
60.20 |
dĒų |
7.407 |
7735 |
ŌņAŌ×ÓµÄŌ×Ó°ė¾¶ĪŖ pm£¬ŹŌ½āŹĶøĆ¾§ĢåŌ×Ó»ÆČČŗÜøßµÄŌŅņ ”£
£ØŅŃÖŖ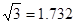 £¬7.407”Ö
£¬7.407”Ö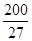 ,1pm=10
,1pm=10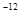 m£©
m£©
£Ø1£©8”¢12£Øø÷1·Ö£©£Ø2£©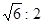 £Ø2·Ö£©
£Ø2·Ö£©
£Ø3£©ĢåŠÄĮ¢·½£Ø2·Ö£© 129.9»ņ130.0£Ø2·Ö£©
ÓÉÓŚA“¦ÓŚdĒų¼Ūµē×Ó¶ą£¬ĒŅ°ė¾¶Š”ĖłŅŌŌ×Ó»ÆČČŗÜøߣØ2·Ö£©
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©T KŅŌĻĀµÄ“æA¾§ĢåĪŖĢåŠÄĮ¢·½½į¹¹£¬ÅäĪ»ŹżŹĒ8£»T KŅŌÉĻµÄ“æA¾§ĢåĪŖĆęŠÄĮ¢·½½į¹¹£¬ÅäĪ»ŹżŹĒ12£»
£Ø2£©ÉčAŌ×Ó°ė¾¶ĪŖr£¬T KŅŌÉĻµÄ“æA¾§Ģå½į¹¹µ„ŌŖµÄ±ß³¤ĪŖa£¬T KŅŌĻĀµÄ“æA¾§Ģå½į¹¹µ„ŌŖµÄ±ß³¤ĪŖb£¬øł¾Ż¶žÕߵĽį¹¹æÉµĆ£ŗ2a2=(4r)2£¬2b2+ b2=(4r)2£¬½ųŅ»²½»Æ¼ņæÉµĆ½į¹ū£»
£Ø3£©Ņ»øö½į¹¹µ„ŌŖŗ¬2øöAŌ×Ó£¬2”Į60.20/(6.02”Į1023)=b3”Į7.407,Ēó³öb£¬ŌŁøł¾Ż£Ø2£©Ēó³öŌ×Ó°ė¾¶r£¬×Ŗ»Æµ„Ī»µĆ³ö“š°ø£»A¼Ūµē×Ó¶ą¶ų°ė¾¶Š”£¬Ō×ÓŗĖĪüŅż¼Ūµē×ÓÄÜĮ¦Ē棬ĖłŅŌŌ×Ó»ÆČČŗÜøß”£
æ¼µć£ŗ±¾Ģā漲龧ĢåµÄÅäĪ»Źż”¢¾§Ģå½į¹¹µÄ¼ĘĖćŗĶŌ×Ó»ÆČČ”£


 ĘߊĒĶ¼ŹéæŚĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
ĘߊĒĶ¼ŹéæŚĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø ³õ֊ѧŅµæ¼ŹŌµ¼ÓėĮ·ĻµĮŠ“š°ø
³õ֊ѧŅµæ¼ŹŌµ¼ÓėĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģÕć½Ź”ĢØ֯֊ѧøßČżµŚŅ»“ĪĶ³Į·»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
ij½šŹō£ØA£©ŌŚTKŅŌĻĀ¾§ĢåµÄ»ł±¾½į¹¹µ„ŌŖČē×óĻĀĶ¼ĖłŹ¾£¬T KŅŌÉĻ×Ŗ±äĪŖÓŅĻĀĶ¼ĖłŹ¾½į¹¹µÄ»ł±¾½į¹¹µ„ŌŖ£¬ŌŚĮ½ÖÖ¾§ĢåÖŠ×īĮŚ½üµÄAŌ×Ó¼ä¾ąĄėĻąĶ¬ ””””””””
””””””””
£Ø1£©ŌŚT KŅŌĻĀµÄ“æA¾§ĢåÖŠ£¬ÓėAŌ×ÓµČ¾ąĄėĒŅ×ī½üµÄAŌ×ÓŹżĪŖ______øö£»ŌŚT KŅŌÉĻµÄ“æA¾§ĢåÖŠ£¬ÓėAŌ×ÓµČ¾ąĄėĒŅ×ī½üµÄAŌ×ÓŹżĪŖ___________£»
£Ø2£©“æA¾§ĢåŌŚ¾§ŠĶ×Ŗ±äĒ°ŗ󣬶žÕß»ł±¾½į¹¹µ„ŌŖµÄ±ß³¤Ö®±ČĪŖ£ØTKŅŌÉĻÓėTKŅŌĻĀÖ®±Č£©___________”£
£Ø3£©ÓŅÉĻĶ¼µÄµÄ¶Ń»ż·½Ź½ĪŖ””””””””””””””””£¬¾²ā¶ØĘä½į¹¹ŗĶŠŌÖŹ²ĪŹżČēĻĀ±ķĖłŹ¾
| ½šŹō | Ļą¶ŌŌ×ÓÖŹĮæ | ·ÖĒų | ĆܶČ/g”¤©M-3 | Ō×Ó»ÆČČ/kJ”¤mol-1 |
| Na | 22.99 | sĒų | 0.960 | 108.4 |
| A | 60.20 | dĒų | 7.407 | 7735 |
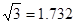 £¬7.407”Ö
£¬7.407”Ö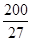 ,1pm=10
,1pm=10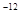 m£©
m£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğÕć½Ź”øßČżµŚŅ»“ĪĶ³Į·»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ij½šŹō£ØA£©ŌŚTKŅŌĻĀ¾§ĢåµÄ»ł±¾½į¹¹µ„ŌŖČē×óĻĀĶ¼ĖłŹ¾£¬T KŅŌÉĻ×Ŗ±äĪŖÓŅĻĀĶ¼ĖłŹ¾½į¹¹µÄ»ł±¾½į¹¹µ„ŌŖ£¬ŌŚĮ½ÖÖ¾§ĢåÖŠ×īĮŚ½üµÄAŌ×Ó¼ä¾ąĄėĻąĶ¬
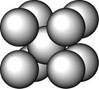 ””””””””
””””””””
£Ø1£©ŌŚT KŅŌĻĀµÄ“æA¾§ĢåÖŠ£¬ÓėAŌ×ÓµČ¾ąĄėĒŅ×ī½üµÄAŌ×ÓŹżĪŖ______øö£»ŌŚT KŅŌÉĻµÄ“æA¾§ĢåÖŠ£¬ÓėAŌ×ÓµČ¾ąĄėĒŅ×ī½üµÄAŌ×ÓŹżĪŖ___________£»
£Ø2£©“æA¾§ĢåŌŚ¾§ŠĶ×Ŗ±äĒ°ŗ󣬶žÕß»ł±¾½į¹¹µ„ŌŖµÄ±ß³¤Ö®±ČĪŖ£ØTKŅŌÉĻÓėTKŅŌĻĀÖ®±Č£©___________”£
£Ø3£©ÓŅÉĻĶ¼µÄµÄ¶Ń»ż·½Ź½ĪŖ””””””””””””””””£¬ ¾²ā¶ØĘä½į¹¹ŗĶŠŌÖŹ²ĪŹżČēĻĀ±ķĖłŹ¾
|
½šŹō |
Ļą¶ŌŌ×ÓÖŹĮæ |
·ÖĒų |
ĆܶČ/g”¤©M-3 |
Ō×Ó»ÆČČ/kJ”¤mol-1 |
|
Na |
22.99 |
sĒų |
0.960 |
108.4 |
|
A |
60.20 |
dĒų |
7.407 |
7735 |
ŌņAŌ×ÓµÄŌ×Ó°ė¾¶ĪŖ””””””””””pm£¬ŹŌ½āŹĶøĆ¾§ĢåŌ×Ó»ÆČČŗÜøßµÄŌŅņ””””””””””””””””””
”””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””£
£ØŅŃÖŖ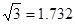 £¬7.407”Ö
£¬7.407”Ö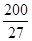 ,1pm=10
,1pm=10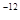 m£©
m£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com