
| �ζ����� | ������Һ���/mL | �������Һ���/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| ��һ�� | 10.00 | 0.50 | 20.40 |
| �ڶ��� | 10.00 | 4.00 | 24.10 |
| V(��)��c(��) |
| V(����) |
| 4.0g |
| 5.0g |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
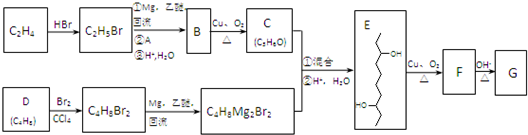
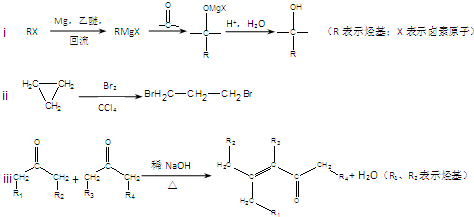
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��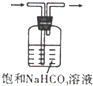 ��ȥCO2�е�HCl���� |
B�� �������ⸯʴ |
C�� �Ʊ����ռ�����NO2���� |
D��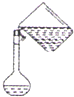 ת����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2H+��aq��+SO42-��aq��+Ba2+��aq��+2OH-��aq��=BaSO4��s��+2H2O��1������H=-57.3 kJ/mol | ||||
B��KOH��aq��+
| ||||
C��C8H18��l��+
| ||||
| D��2C8H18��g��+25O2��g��=16CO2��g��+18 H2O��1������H=-5518 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | ʵ����� | ʵ������ | ���� |
| A | ���������̼������Һ�� | �����ݲ��� | �ȵķǽ����Ա�̼ǿ |
| B | ����ҺX�м���ϡ���ᣬ������������ɫ����ͨ�����ʯ��ˮ�� | ���ɰ�ɫ���� | ��ҺX��һ������CO32-��HCO3- |
| C | �������Һ����ϡH2SO4�����ȼ����ӣ���ȴ���ټ��� ����Cu��OH��2������ | û�к�ɫ�������� | ����û��ˮ��������� |
| D | ȡ���õ��̷���FeSO4?7H2O������ˮ������KSCN��Һ | ��Һ��ΪѪ��ɫ | �̷����ֻ�ȫ�������� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 ���Ǻϳ�APM��ԭ��֮һ��APM�Ľṹ��ʽ��ͼ1��ʾ��
���Ǻϳ�APM��ԭ��֮һ��APM�Ľṹ��ʽ��ͼ1��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��BaCl2������ |
| B����֧�Թܵİ�ɫ�������������ᱵ |
| C��SO2�л�ԭ�Ժ������������ͨ�� |
| D������˵���������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com