
��9�֣�����ͼ��Ԫ�����ڱ��Ķ����ڲ��֣����a��h����8�ֳ���Ԫ�ء�
| | IA[ | O�� | ||||||
| ��һ���� | a | IIA | IIIA | IVA | VA | VIA | VIIA | |
| �ڶ����� | | | | b | c | d | | |
| �������� | e | | f[ | | | g | h | |
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| IA | O�� | |||||||||
| ��һ���� | a | IIA | IIIA | IVA | VA | VIA | VIIA | |||
| �ڶ����� | b | c | d | |||||||
| �������� | e | f | g | h | ||||||


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�� ���� |
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� | �� |


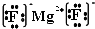
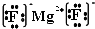
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
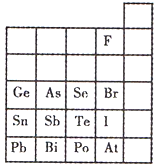 Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�
Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�





�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�





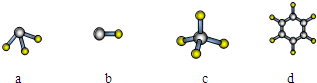
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com