
 CO(g)+H2(g)£»
CO(g)+H2(g)£»(3)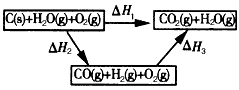
 Ņ»ÅµŹéŅµŹī¼Ł×÷ŅµæģĄÖ¼ŁĘŚŌĘÄĻĆĄŹõ³ö°ęÉēĻµĮŠ“š°ø
Ņ»ÅµŹéŅµŹī¼Ł×÷ŅµæģĄÖ¼ŁĘŚŌĘÄĻĆĄŹõ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗѧĻ°Öܱؔ”»Æѧ””ČĖ½ĢæĪ±źø߶ž°ę(Ń”ŠŽ4)””2009£2010ѧğ””µŚ3ĘŚ””×ܵŚ159ĘŚ ČĖ½ĢæĪ±ź°ę(Ń”ŠŽ4) ĢāŠĶ£ŗ022
½«Ćŗ×Ŗ»ÆĪŖĖ®ĆŗĘųŹĒĶعż»Æѧ·½·Ø½«Ćŗ×Ŗ»ÆĪŖ½ą¾»Č¼ĮĻµÄ·½·ØÖ®Ņ»£®Ćŗ×Ŗ»ÆĪŖĖ®ĆŗĘųµÄÖ÷ŅŖ·“Ó¦ĪŖ£ŗ
C£«H2O
ŅŃÖŖ£ŗ
1 mol””H2O(g)×Ŗ»ÆĪŖ1 mol””H2O(l)·Å³ö44.0 kJµÄČČĮ森»Ų“šĻĀĮŠĪŹĢā£ŗ(1)Š“³öC²»ĶźČ«Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½£ŗ________£®
(2)ĻÖÓŠH2»ņCOĪŖČ¼ĮĻĢį¹©ČČÄÜ£¬ÄćČĻĪŖÓ¦øĆŃ”Ōń________(ĢīŠ“ŠņŗÅ)£»
a£®H2””b£®CO””c£®¾łæÉŅŌĄķÓÉŹĒ________£®
(3)¼×Ķ¬Ń§øł¾Ż1 mol””CO(g)ŗĶ1 mol””H2(g)ĶźČ«Č¼ÉշųöµÄČČĮæ×ÜŗĶ±Č1 mol””C(s)ĶźČ«Č¼ÉշųöµÄČČĮæ¶ą£¬ČĻĪŖ£ŗ”°ĆŗČ¼ÉÕŹ±¼ÓÉŁĮæĖ®£¬æÉŅŌŹ¹ĆŗČ¼Éշųöøü¶ąµÄČČĮ森”±ŅŅĶ¬Ń§øł¾ŻøĒĖ¹¶ØĀÉŠ“³öĻĀĮŠŃ»·Ķ¼£ŗ
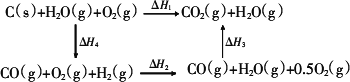
¾Ż“Ė£¬ŅŅĶ¬Ń§ČĻĪŖ£ŗ”°½«Ćŗ×Ŗ»ÆĪŖĖ®ĆŗĘųŌŁČ¼ÉշųöµÄČČĮæÓėÖ±½ÓČ¼ÉÕĆŗ·Å³öµÄČČĮæŅ»Ńł¶ą£¬¶ų½«Ćŗ×Ŗ»ÆĪŖĖ®ĆŗĘų»įŌö¼ÓÉś²ś»·½Ś”¢Ōö¼ÓĻūŗÄ£¬Ņņ“Ė£¬½«Ćŗ×Ŗ»ÆĪŖĖ®ĆŗĘųµĆ²»³„Ź§£®”±ĒėÄć¶Ō¼×”¢ŅŅĮ½Ķ¬Ń§µÄ¹ŪµćµÄÕżČ·ŠŌ½ųŠŠĘĄ¼Ū²¢²ūŹöÄćµÄĄķÓÉ£ŗ
________£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com