
��15�֣�ʵ������Ҫ0.1mol/LNaOH��Һ480mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⡣
��1������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_________������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ���������_____ _____��
A B C D E
��2��������ƿ��ʹ�÷����У����в�������ȷ����____________
A��ʹ������ƿǰ�����Ƿ�©ˮ
B��������Һʱ����������ǹ��壬�ѳƺõĹ�����ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ��̶���1~2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ�
C��������Һʱ����������Һ�壬����Ͳȡ�����ò�����������������ƿ�У�������ˮ���̶���1~2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ�
D���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȡ�
��3�����ݼ�����������ƽ��ȡ������Ϊ g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ�� 0.1mol/L������ڡ���С�ڡ����ڡ�����
��4�����ݼ����֪��������Ͳ��ȡ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ mL������1λС���������ʵ������15mL��20mL��50mL��Ͳ��Ӧѡ�� mL��Ͳ��á�
��5������������Һ�����У����в�����������ƫ�ߵ��� ������ţ�
��δϴ���ձ���������
��δ��ȴ�����¾�ת�Ƶ�����ƿ����
������ƿ�����������������ˮ
��15�֣���1��B��D��2�֣���1�֣���������500mL����ƿ��2�֣���1�֣�
(2) B��C��2�֣���1�֣�
��3��2.0��2�֣� С�ڣ�2�֣�
(4) 13.6��2�֣� 15��1�֣�
��5���ڣ�2�֣�
��������
�����������1��������Һ��Ҫ���������ձ���������������ƿ����ͷ�ιܡ���Ͳ������ƽ�ȣ�����Ҫ��ƿ�ͷ�Һ©������ѡBD������480mL����500mL����Һ����Ҫ500mL������ƿ������ʱ��Ҫ��ͷ�ιܣ����Ի���Ҫ�IJ�����������500mL������ƿ����ͷ�ιܣ�
��2��A.����ƿʹ��ǰ�������Ƿ�©ˮ����ȷ��B��������Һʱ�����������ǹ��廹��Һ�壬������������ƿ��ֱ���ܽ⣬Ӧ���ձ����ܽ����ȴ�����º�����������ƿ�У�����C������B�ķ���������D�����ݺǺ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȣ���ȷ����ѡBC��
��3������500mL��0.1mol/LNaOH��Һ��Ҫ�������Ƶ�������0.5L��0.1mol/L��40g/mol=2.0g��������ʱ���ӿ̶��ߣ�ʹ������Һ���������500mL����������ҺŨ��С��0.1mol/L��
��4���������ʵ���Ũ������Һ���������Ĺ�ϵ����������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������ʵ���Ũ����1000��1.84��98%/98=18.4mol/L��500mL0.5mol/L��������Һ����������ʵ�����0.5L��0.5mol/L=0.25mol��������ҪŨ����������0.25mol/18.4mol/L=0.0136L=13.6mL������ѡ����һ����ȡ����С������Ͳ�����С������ѡ��15mL����Ͳ��
��5��δϴ���ձ�����������ʹ����ƫС��������Һ��Ũ��ƫ�ͣ�δ��ϡ�ͺ��H2SO4��Һ��ȴ�����¾�ת�Ƶ�����ƿ�У�ʹ����ʱ��Һ���¶�ƫ�ߣ���ȴ��������٣�Ũ��ƫ�ߣ�����ƿ�к�����������ˮ���Խ����Ӱ�죬���Դ�ѡ�ڡ�
���㣺������Һ���ƵIJ��������㣬���ķ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��[ͬ��]2014���˽̰���л�ѧѡ��һ��һ��1.3��ϰ���������棩 ���ͣ�ѡ����
��2014?ɽ��ģ�⣩����˵���У�����ȷ���ǣ� ��
A.�л���������ÿ��̼ԭ������γ�4�����ۼ�
B.��֬�����ۡ���������һ�������¶��ܷ���ˮ�ⷴӦ
C.����ˮ�ȿ��Լ���������ϩ��Ҳ���Գ�ȥ�����е���ϩ
D.��ϩ�ͱ����ܷ���������Ӧ��˵����ϩ�ͱ������о���̼̼˫��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��[ͬ��]2014���˽̰���л�ѧѡ��һ��һ��1.1��ϰ���������棩 ���ͣ�ѡ����
��2014?�㽭ģ�⣩����˵����ȷ���ǣ� ��
A.�ڵ�����Һ�м�������ϡ�����ȣ��ټ���������������ͭ����Һ���ȣ���ɫ������˵������δˮ��
B.˫��ˮ���ȶ����ֽ⣮�������������������ֽ�����
C.ʳ�����Ậ���ⶨʵ���У���25mL��Һ����ȡ����ʳ��25mL����ƿ��������������Һֱ�Ӳⶨ
D.��ͭп�缫�õ��������ֱ��������ʢ������ͭ��Һ���ձ����м���������������ԭ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�츣��ʡ��һ��ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵���д�����ǣ� ��
A����2 L 1 mol��L��1��NaCl��Һ��ȡ��10 mL����Ũ������1 mol��L��1
B���Ƴ�0.5 L 1 mol��L��1�����ᣬ���״�����Ȼ�������11.2 L
C��1 L 1 mol��L��1 BaCl2��Һ�У�Ba2����Cl������Ϊ3��6.02��1023
D��10g 98�����ᣨ�ܶ�Ϊ1.84g��cm��3����10mL 18.4 mol��L��1�����Ũ���Dz�ͬ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�츣��ʡ��һ��ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����Լ��ı��治��ȷ���� �� ��
A��Ư�۱����ܷⱣ�� B��������ˮ����ɫ�Ĺ��ƿ����
C�����������Ʊ�����ú���� D��Һ����һ��ˮ������ܷⱣ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ֣�ݶ�����ѧ�ڸ�һ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������¼�����Ӧ��
��Cl2��2KBr��2KCl��Br2 ��Br2��2KI��2KBr��I2 �ж���������ǿ������˳���ǣ� ��
A��I2��Br2��Cl2 B��Cl2��Br2��I2 C��Br2��I2��Cl2 D��Cl2��I2��Br2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ֣�ݶ�����ѧ�ڸ�һ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ�뻯ѧ��Ӧ��ʵһ�µ��ǣ� ��
A������ͭ��Һ������������Һ��Ӧ��Cu2+��2OH����Cu(OH)2��
B��ʯ��ʯ�������CO32����2H+��H2O��CO2��
C��������Һ������������Һ��Ӧ��H+��OH����H2O
D��Cu��AgNO3��Һ��Ӧ��Cu��Ag+��Cu2+��Ag
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
��9��Ϊ�˽����������Ƶ��Ȼ����ᴿ�����Ƶô������Ȼ�����Һ��ijѧ���������ʵ�飺
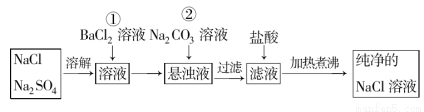
��1���������ܷ������ᱵ��Һ��_____ (�ܻ��˵������__________________��
��2�����в����ٺ�����ж�SO42-�ѳ�����������_____________ ________________��
��3�������ڵ�Ŀ����_______________��Ϊʲô���ȹ��˶����̼������Һ�������� ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�콭��ʡ��һ���£���ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��NAΪ�����ӵ�������ֵ�������й�������ȷ����
A����״���£�1L������ȫȼ�պ������ɵ���̬����ķ�����Ϊ
B��100 mL 0.1 mol/L CH3COOH��Һ������п��Ӧ�����ɵ�����������Ϊ0.01 NA
C��0.1 mol CH4����������ΪNA
D��0.5mol C2H4�к��е�C=C˫����ΪNA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com