
(16��)���淴Ӧ2 Cl2 (g)+2H2O(g)  4HCl(g)+O2
(g) ��H��0����һ�������´ﵽƽ��ֱ��ȡ���д�ʩ���Իش�(1-4�����������С�������䡱)
4HCl(g)+O2
(g) ��H��0����һ�������´ﵽƽ��ֱ��ȡ���д�ʩ���Իش�(1-4�����������С�������䡱)
(1)�����¶ȣ�Cl2��ת����__ _____��ƽ�ⳣ��K
(2)��������������䣬����He����HCl�����ʵ���______________
(3) ��������������䣬����Cl2����Cl2��ת����_________��ƽ�ⳣ��K
(4)�����º��ݣ���Ӧ��Cl2��H2O��ʼ��ƽ�⣬������仯�����У�ƽ����Է�������____��
��5����Ӧ��ϵ�м�������Է�Ӧ���Ƿ���Ӱ�죿______________________��ԭ����_______��
(1)��С ��С (2) ���� (3) ��С ���� (4) ��С
��5��ûӰ�죻����ֻ�ܸı仯ѧ��Ӧ�����ʺ�;�������ܸı仯ѧ��Ӧʼ̬����̬����������˶Է�Ӧ��û��Ӱ��
��������������������Ի�ѧƽ���Ӱ�졣
���ݷ�Ӧʽ��֪���÷�Ӧ���������ġ����ȵĿ��淴Ӧ�����Խ����¶�ƽ��������Ӧ�����ƶ���ת���ʼ�С��ƽ�ⳣ��ֻ���¶��йأ�����ƽ�ⳣ����С����������������䣬����He�����ʵ�Ũ�Ȳ��䣬ƽ�ⲻ�ƶ����Ȼ�������ʵ������䡣����������Ũ�ȣ�������ת���ʼ�С��ƽ�ⳣ�����䡣�����Cl2��H2O��ʼ��ƽ�⣬�������ƽ����Է��������ǻ���������������ʵ����ı�ֵ������ƽ����Է���������С�������ܸı䷴Ӧ�Ļ�ܣ�����Ӧ�����������������Dz��䣬��Ӧ���Dz���ġ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��)���淴Ӧ2 Cl2 (g)+2H2O(g) 4HCl(g)+O2(g) ��H��0����һ�������´ﵽƽ��ֱ��ȡ���д�ʩ���Իش�(1-4�����������С�������䡱)
(1)�����¶ȣ�Cl2��ת����__ _____��ƽ�ⳣ��K
(2)��������������䣬����He����HCl�����ʵ���______________
(3) ��������������䣬����Cl2����Cl2��ת����_________��ƽ�ⳣ��K
(4)�����º��ݣ���Ӧ��Cl2��H2O��ʼ��ƽ�⣬������仯�����У�ƽ����Է�������____��
��5����Ӧ��ϵ�м�������Է�Ӧ���Ƿ���Ӱ�죿______________________��ԭ����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ�䶨�ص�һ��ѧ�߶�5���¿���ѧ�Ծ����������� ���ͣ������
(16��)���淴Ӧ2 Cl2 (g)+2H2O(g)  4HCl(g)+O2 (g) ��H��0����һ�������´ﵽƽ��ֱ��ȡ���д�ʩ���Իش�(1-4�����������С�������䡱)
4HCl(g)+O2 (g) ��H��0����һ�������´ﵽƽ��ֱ��ȡ���д�ʩ���Իش�(1-4�����������С�������䡱)
(1)�����¶ȣ�Cl2��ת����__ _____��ƽ�ⳣ��K
(2)��������������䣬����He����HCl�����ʵ���______________
(3) ��������������䣬����Cl2����Cl2��ת����_________��ƽ�ⳣ��K
(4)�����º��ݣ���Ӧ��Cl2��H2O��ʼ��ƽ�⣬������仯�����У�ƽ����Է�������____��
��5����Ӧ��ϵ�м�������Է�Ӧ���Ƿ���Ӱ�죿______________________��ԭ����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��Ϫһ�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��ÿ��2�֣���16�֣�ij�о���ѧϰС��Ϊ�ϳ�1���������������ϵ�֪һ���ϳ�·�ߣ�
CH3CH===CH2��CO��H2 CH3CH2CH2CHO
CH3CH2CH2CHO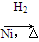 CH3CH2CH2CH2OH��
CH3CH2CH2CH2OH��
CO���Ʊ�ԭ����HCOOH CO����H2O������Ƴ�ԭ�������Ʊ�װ��(��ͼ)��
CO����H2O������Ƴ�ԭ�������Ʊ�װ��(��ͼ)��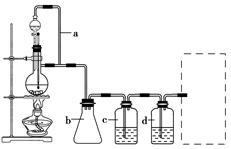
����д���пհף�
(1)ʵ��������п����ϡ���ᡢϡ���ᡢŨ���ᡢ2������������ѡ����ʵ��Լ��Ʊ���ϩ��д����ѧ����ʽ�� ____________________________________________________________________________��
(2)��������װ���Ʊ�H2�������߿��ڻ����ռ�����H2��װ��ͼ��
(3)�Ʊ�ϩʱ������������SO2��CO2��ˮ��������С���������Լ��������������壬�������ͨ���Լ���˳����________(�����)
�ٱ���Na2SO3��Һ��������KMnO4��Һ����ʯ��ˮ������ˮCuSO4����Ʒ����Һ
(4)�ϳ�����ȩ�ķ�ӦΪ������ȵĿ��淴Ӧ��Ϊ����Ӧ���ʺ����ԭ������ת���ʣ�����ΪӦ�ò��õ����˷�Ӧ������________��
a�����¡���ѹ������ b���ʵ����¶ȡ���ѹ������
c�����¡���ѹ������ d���ʵ����¶ȡ���ѹ������
(5)����ȩ��������õ�����������ȩ��1��������Ʒ��Ϊ����1����������С���������֪����R��CHO��NaHSO3(����)��RCH(OH)SO3Na�� ���ڷе㣺����34�棬1������118�棬����Ƴ������ᴿ·�ߣ�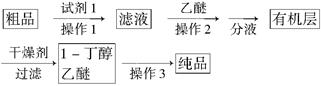
�Լ�1Ϊ________������1Ϊ________������2Ϊ________������3Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��ÿ��2�֣���16�֣�ij�о���ѧϰС��Ϊ�ϳ�1���������������ϵ�֪һ���ϳ�·�ߣ�
CH3CH===CH2��CO��H2 CH3CH2CH2CHO
CH3CH2CH2CHO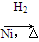 CH3CH2CH2CH2OH��
CH3CH2CH2CH2OH��
CO���Ʊ�ԭ����HCOOH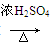 CO����H2O������Ƴ�ԭ�������Ʊ�װ��(��ͼ)��
CO����H2O������Ƴ�ԭ�������Ʊ�װ��(��ͼ)��

����д���пհף�
(1)ʵ��������п����ϡ���ᡢϡ���ᡢŨ���ᡢ2������������ѡ����ʵ��Լ��Ʊ���ϩ��д����ѧ����ʽ�� ____________________________________________________________________________��
(2)��������װ���Ʊ�H2�������߿��ڻ����ռ�����H2��װ��ͼ��
(3)�Ʊ�ϩʱ������������SO2��CO2��ˮ��������С���������Լ��������������壬�������ͨ���Լ���˳����________(�����)
�ٱ���Na2SO3��Һ��������KMnO4��Һ����ʯ��ˮ������ˮCuSO4����Ʒ����Һ
(4)�ϳ�����ȩ�ķ�ӦΪ������ȵĿ��淴Ӧ��Ϊ����Ӧ���ʺ����ԭ������ת���ʣ�����ΪӦ�ò��õ����˷�Ӧ������________��
a�����¡���ѹ������ b���ʵ����¶ȡ���ѹ������
c�����¡���ѹ������ d���ʵ����¶ȡ���ѹ������
(5)����ȩ��������õ�����������ȩ��1��������Ʒ��Ϊ����1����������С���������֪����R��CHO��NaHSO3(����)��RCH(OH)SO3Na�� ���ڷе㣺����34�棬1������118�棬����Ƴ������ᴿ·�ߣ�
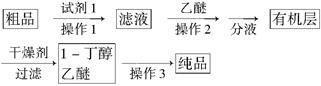
�Լ�1Ϊ________������1Ϊ________������2Ϊ________������3Ϊ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com