
 ����Ԫ��ͨ��ת��Ϊ������Σ�笠����ӵĵ���ʽΪ
����Ԫ��ͨ��ת��Ϊ������Σ�笠����ӵĵ���ʽΪ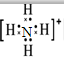 ��
�� ���� ��1��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų��������ȣ��������������ϻ�ѧ����ʽ�����Ӧ�����ʱ䣬��ע���ʾۼ�״̬д���Ȼ�ѧ����ʽ��
��2�����ݻ��ϼ۱仯�жϱ���ԭ��Ԫ�أ�Ȼ���ϸ�Ԫ��ԭ�ӵ�ԭ������д����ԭ�ӽṹʾ��ͼ��笠�����Ϊ�����ӣ���Ҫ���������ɼ��������ӣ�
��� �⣺��1��0.2 molҺ̬����������Һ̬˫��ˮ��Ӧʱ�ų�128kJ��������1molҺ̬�º������ⷴӦ���ȣ�128kJ��$\frac{1mol}{0.2mol}$=640kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��N2H4��l��+2H2O2��l��=N2��g��+4 H2O��g����H=-640kJ/mol
�ʴ�Ϊ��N2H4��l��+2 H2O2��l��=N2��g��+4H2O��g����H=-640kJ/mol��
��2����ӦN2H4��l��+2H2O2��l��=N2��g��+4H2O��g���У�N2H4��-2��NԪ�ػ��ϼ����߱�������H2O2��-1��OԪ�ػ��ϼ۽��ͱ���ԭ�����е�Ԫ��ΪO��Oԭ�ӵ�ԭ������Ϊ8����ԭ�ӽṹʾ��ͼΪ�� ��笠�����Ϊ���������ӣ�����ʽ����Ҫ���������ɼ�ԭ�ӵ��������ӣ������ʽΪ
��笠�����Ϊ���������ӣ�����ʽ����Ҫ���������ɼ�ԭ�ӵ��������ӣ������ʽΪ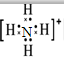 ��
��
�ʴ�Ϊ��O�� ��
��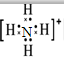 ��
��
���� ���⿼���˷�Ӧ�����ʱ��Ӧ�ã���Ŀ�Ѷ��еȣ��漰�Ȼ�ѧ����ʽ��д��������ԭ��Ӧ������ʽ��ԭ�ӽṹʾ��ͼ��֪ʶ����ȷ�Ȼ�ѧ����ʽ����дԭ��Ϊ���ؼ���ע�����ճ�����ѧ����ĸ����ʾ����������������ѧ���Ĺ淶����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
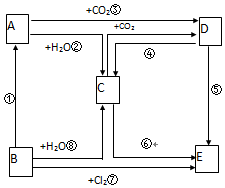 ��ͼ��ʾ����A��E�������ʵ��ת����ϵ������AΪ����ɫ���壬BΪ���ʣ�
��ͼ��ʾ����A��E�������ʵ��ת����ϵ������AΪ����ɫ���壬BΪ���ʣ� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�| �ζ� ���� | �������������� Һ�����/mL | 0.1000mol•L+1����������mL�� | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| ��һ�� | 25.00 | 0.00 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 |
| ������ | 25.00 | 0.22 | 26.31 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶�/�� | 200 | 300 | 400 |
| K | 1.0 | 0.85 | 0.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 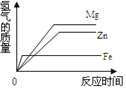 | B�� | 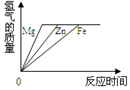 | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͭ���ڷ����ڳ�ʪ�Ŀ������������⣬����Cu��OH��2 | |
| B�� | ͭ˿��������ȼ������CuCl2������Ӧ����Cu2S��˵��������Cl2��S | |
| C�� | CuO����Cu2O�ȶ�������������CuO���Էֽ�����Cu2O������ | |
| D�� | ��ɫ��CuSO4•5H2O�������ȷֽ�ת��Ϊ��ɫ��CuSO4 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com