
20��ʱ��20mL 0.1mol��L��1������Һ�в��ϵ���0.1mol��L��1NaOH(aq)����ҺpH�仯��ͼ��ʾ���˹�������Һ������Ũ�ȵĹ�ϵ������� 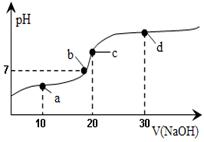
| A��a�㣺c(Na+)��c(CH3COO��)��c(H+)��c(OH��) |
| B��b�㣺c(Na+) = c(CH3COO��)��c(H+) = c(OH��) |
| C��c�㣺c(H+) = c(CH3COOH) + c(OH��) |
| D��d�㣺c(Na+)��c(CH3COO��)��c(OH��)��c(H+) |
CD
�������������A��a��ʱ�����������ҺΪCH3COOH��CH3COONa�Ļ�����Һ�����ԣ�����c��H+����c��OH-������Һ�ʵ����ԣ�c��Na+��+c��H+��=c��CH3COO-��+c��Cl-��+c��OH-��������c��Na+����c��CH3COO-������A��ȷ�� B��������Һ����غ��֪��Һ��Ӧ����c��Na+��+c��H+��=c��CH3COO-��+c��OH-������Һ�����ԣ�Ӧ��c��H+��=c��OH-������c��Na+��=c��CH3COO-������B��ȷ�� C��c��ʱ����Һ�ʼ��ԣ�Ӧ��c��H+����c��OH-������C���� D��d��ΪNaOH��CH3COONa�Ļ�����Һ�ʼ��ԣ�����CH3COO-��������ˮ�⣬����c��Na+����c��OH-����c��CH3COO-����c��H+������D����ѡCD��
���㣺���⿼������ϵ��жϺ�����Ũ�ȴ�С�Ƚϣ�����ʱע��a��b��c��d����Һ����ɣ�����������ʵĵ��������ˮ����ص㣬��Ŀ�Ѷ��еȣ�


 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����ݾ�Ϊ100mL0.5mol��L-1NaHCO3��Һ�У��ֱ�������������ᡢCa(OH)2���塢NaAlO2���壨������Һ����仯������������Һ��c(CO32��)�ı仯����Ϊ
| A�������������� | B�������������� |
| C������С����С | D����С������С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
һ���¶��£�����AgCl(s)�ı���AgCl��Һ�м�ˮ������������ȷ���ǣ� ��
| A��AgCl���ܽ������ | B��AgCl���ܽ������Ksp���� |
| C��C��Ag+������ | D��AgCl���ܽ�ȡ�Ksp������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����£��ڵ������pH=0�����ᡢ��0.01mol/L NaOH��Һ����pH=10�Ĵ�����Һ����pH=5��NH4Cl��Һ�У�ˮ����̶ȵĴ�С˳����
| A���٣��ڣ��ۣ��� | B���ڣ��٣��ܣ��� | C���ۣ��ܣ��ڣ��� | D���ܣ��ۣ��ڣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����£������Ϊ10 mL�����ʵ���Ũ�Ⱦ�Ϊ0.1 mol/L������ʹ���Ļ����Һ�е���0.1 mol��L NaOH��Һ������˵���������( )
| A������NaOH��Һ����Һһ�����㣺 c(H+)+c(Na+)=c(OH-)+c(C1-)+c(CH3COO-) |
| B������10 mLNaOH��Һʱ����Һ����(��Һ����仯���Բ���)�� c(CH3COO-)+c(CH3COOH)="0.05" mol/L |
| C������15 mLNaOH��Һʱ�������ԣ���Һ���㣺 c(Na+)>c(C1-)>c(CH3COO-)>c(CH3COOH)>c(H+)>c(OH-) |
| D����������NaOH��Һʱ����Һ�з������кͷ�Ӧ���Ȼ�ѧ����ʽ��ʾΪ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����£�0.1 mol/L��ˮ��Һ�� ��1��10-8�����������������
��1��10-8�����������������
| A������Һ�������ӵ�Ũ�ȣ�c(H+ ) �� 1��10-9 mol/L |
| B��0.1 mol/L��ˮ��Һ��0.1 mol/L HCl��Һ�������Ϻ�������Һ�У� c(NH4+ ) + c(H+ ) �� c(Cl- ) + c(OH-) |
| C��0.1 mol/L�İ�ˮ��Һ��0.05 mol/L H2SO4��Һ�������Ϻ�������Һ�У� c(NH4+ ) + c(NH3) + c(NH3��H2O) �� 2c(SO42-) |
| D��Ũ�Ⱦ�Ϊ0.1 mol/L��NH3��H2O��NH4Cl��Һ�������Ϻ�����Һ�ʼ��ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����£���pH=3�����ᡢ���ᡢ���ᣨ����HAc�ĵ����Ϊ1%��������ͬ�������Һ�����������������
| A���ⶨ�䵼��������ͬ |
| B����������п�۷�Ӧ����ʼ������ͬ |
| C����������п�۷�Ӧ���������������Ϊ1��1��100 |
| D����ͬŨ������������Һ��Ӧ����������������Һ�����Ϊ1��2��100 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������pH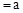 ��CH3COOHϡ��Һ��pH
��CH3COOHϡ��Һ��pH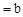 ��NaOHϡ��Һ�������ϣ������жϴ���
��NaOHϡ��Һ�������ϣ������жϴ���
���ǣ� ��
A������Ϻ�pH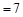 ���� ����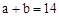 |
| B����Ӧ�����У�CH3COOH�ĵ�������� |
C������Ϻ�pH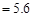 ����c ����c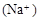 ��c ��c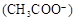 |
D������Ϻ�CH3COOH��NaOHǡ����ȫ��Ӧ����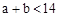 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪����ͬ����������ǿ��˳��Ϊ��CH3COOH>H2CO3>HCN>HCO3�������б�������ȷ����
| A��25��ʱ�������ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH(Na2CO3)��pH(NaCN)��pH(NaHCO3)��pH(CH3COONa) |
| B��a mol��L��1 HCN��Һ��bmol��L��1 NaOH��Һ�������Ϻ�������Һ�У�c(Na+)��c(CN��)����aһ������b |
| C��0.1mol��L��1��Na2CO3��Һ�У�c(OH��)��c(H+)��c(HCO3��)��c(H2CO3) |
D��25��ʱ��pH��4.75��Ũ�Ⱦ�Ϊ0.1mol��L��1��CH3COOH��CH3COONa�����Һ��c( )+c( )+c(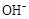 )<c(CH3COOH)+c(H+) )<c(CH3COOH)+c(H+) |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com