

| vÕżA |
| vÄęB |
| 1 |
| 1 |
| 2 |
| 1 |


 ĢŅĄīĪÄ»ÆæģĄÖŹī¼ŁĪäŗŗ³ö°ęÉēĻµĮŠ“š°ø
ĢŅĄīĪÄ»ÆæģĄÖŹī¼ŁĪäŗŗ³ö°ęÉēĻµĮŠ“š°ø ÓÅŠćÉśæģĄÖ¼ŁĘŚĆæŅ»ĢģČ«ŠĀŗ®¼Ł×÷Ņµ±¾ĻµĮŠ“š°ø
ÓÅŠćÉśæģĄÖ¼ŁĘŚĆæŅ»ĢģČ«ŠĀŗ®¼Ł×÷Ņµ±¾ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)Čē¹ūÓĆĻĀĮŠĒéæöµÄÅä±Č×÷ĘšŹ¼Īļ£¬ŌŚĶ¬ŃłµÄČŻĘ÷ŗĶĪĀ¶ČĻĀ“ļµ½Ę½ŗā£¬CŌŚĘ½ŗā»ģŗĻĘųĢåÖŠµÄĢå»ż·ÖŹżČŌĪŖn%µÄŹĒ______________________”£
A.4 mol A+2 mol B
B.6 mol C+1 mol D
C.1 mol A+0.5 mol B+1.5 mol C+0.5 mol D
D.2 mol A+1 mol B+3 mol C+1 mol D
E.2 mol A+1 mol B+3 mol C+0.5 mol D
(2)ČōĪ¬³ÖøĆĢāĢõ¼ž²»±ä£¬½ö“ÓÉś³ÉĪļæŖŹ¼Åä±Č£¬ŅŖĒó“ļµ½Ę½ŗāŹ±£¬CµÄĪļÖŹµÄĮæČŌĪŖW mol£¬ŌņDµÄĘšŹ¼ĪļÖŹµÄĮæn(D)Ó¦Āś×ćµÄĢõ¼žŹĒ_________________£ØÓĆŗ¬WµÄ“śŹżŹ½±ķŹ¾£©”£
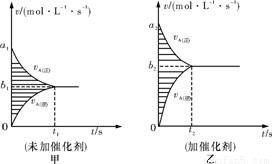
(3)“Ė·“Ó¦µÄv-tĶ¼ĻóČē¼×Ķ¼£¬ČōĘäĖūĢõ¼ž¶¼²»±ä£¬Ö»ŹĒŌŚ·“Ó¦Ē°¼ÓČėŗĻŹŹµÄ“߻ƼĮ£¬ŌņĘäv-tĶ¼ĻóČēŅŅĶ¼£¬ĒėÓĆ”°£½”¢£¼”¢£¾”±ĢīæÕ£ŗ¢Ła1________________a2£»¢Śb1________________b2£»¢ŪĮ½Ķ¼ÖŠŅõÓ°²æ·ÖĆ껿£ŗ¼×_______ŅŅ”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŅ»øöČŻ»ż¹Ģ¶Ø²»±äµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦£ŗ2A(g)£«B(g) 3C(g)£«D(s)£¬ŅŃÖŖ½«2mol AŗĶ1molB³äČėøĆČŻĘ÷ÖŠ£¬·“Ó¦ŌŚÄ³ĪĀ¶ČĻĀ“ļµ½Ę½ŗāŹ±£¬CµÄĪļÖŹµÄĮæĪŖWmol£¬CŌŚĘ½ŗā»ģŗĻĘųĢåÖŠµÄĢå»ż·ÖŹżĪŖn%”£
(1)Čē¹ūÓĆĻĀĮŠĒéæöµÄÅä±Č×÷ĘšŹ¼Īļ£¬ŌŚĶ¬ŃłµÄČŻĘ÷ŗĶĪĀ¶ČĻĀ“ļµ½Ę½ŗā£¬Ęä½į¹ūŅ»¶ØŹĒ£ŗCµÄĪļÖŹµÄĮæĪŖ2Wmol£¬CŌŚĘ½ŗā»ģŗĻĘųĢåÖŠµÄĢå»ż·ÖŹżČŌĪŖn%µÄŹĒ”””””””””£
A.4molA£«2mol B
B.6molC£«1mol D
C.1molA£«0.5mol B£«1.5mol C£«0.5mol D
D.2molA£«1mol B£«3mol C£«1mol D
E.2molA£«1mol B£«3mol C£«2mol D
(2)ČōĪ¬³ÖøĆĢāĢõ¼ž²»±ä£¬½ö“ÓÉś³ÉĪļæŖŹ¼Åä±Č£¬ŅŖĒó“ļµ½Ę½ŗāŹ±£¬CµÄĪļÖŹµÄĮæČŌĪŖWmol£¬ŌņDµÄĘšŹ¼ĪļÖŹµÄĮæn(D)Ó¦Āś×ćµÄĢõ¼žŹĒ””””””””””””””(ÓĆŗ¬WµÄ“śŹżŹ½±ķŹ¾)”£
(3)“Ė·“Ó¦µÄv£tĶ¼ĻóČē¼×Ķ¼£¬ČōĘäĖūĢõ¼ž¶¼²»±ä£¬Ö»ŹĒŌŚ·“Ó¦Ē°¼ÓČėŗĻŹŹµÄ“߻ƼĮ£¬ŌņĘäv£tĶ¼ĻóČēŅŅĶ¼£¬ĒėÓĆ”°£½”¢£¼”¢£¾”±ĢīæÕ£ŗ
¢Ła1””””””a2£»¢Śb1””””””b2£»¢ŪĮ½Ķ¼ÖŠŅõÓ°²æ·ÖĆ껿£ŗ¼×””””””ŅŅ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğŗžÄĻŹ”³¤É³ŹŠøßČżµŚĮł“ĪŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
ŌŚŅ»øöČŻ»ż¹Ģ¶Ø²»±äµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦£ŗ2A(g)£«B(g)  3C(g)£«D(s)£¬ŅŃÖŖ½«2mol AŗĶ1mol
B³äČėøĆČŻĘ÷ÖŠ£¬·“Ó¦ŌŚÄ³ĪĀ¶ČĻĀ“ļµ½Ę½ŗāŹ±£¬CµÄĪļÖŹµÄĮæĪŖWmol£¬CŌŚĘ½ŗā»ģŗĻĘųĢåÖŠµÄĢå»ż·ÖŹżĪŖn%”£
3C(g)£«D(s)£¬ŅŃÖŖ½«2mol AŗĶ1mol
B³äČėøĆČŻĘ÷ÖŠ£¬·“Ó¦ŌŚÄ³ĪĀ¶ČĻĀ“ļµ½Ę½ŗāŹ±£¬CµÄĪļÖŹµÄĮæĪŖWmol£¬CŌŚĘ½ŗā»ģŗĻĘųĢåÖŠµÄĢå»ż·ÖŹżĪŖn%”£
(1)Čē¹ūÓĆĻĀĮŠĒéæöµÄÅä±Č×÷ĘšŹ¼Īļ£¬ŌŚĶ¬ŃłµÄČŻĘ÷ŗĶĪĀ¶ČĻĀ“ļµ½Ę½ŗā£¬Ęä½į¹ūŅ»¶ØŹĒ£ŗCµÄĪļÖŹµÄĮæĪŖ2Wmol£¬CŌŚĘ½ŗā»ģŗĻĘųĢåÖŠµÄĢå»ż·ÖŹżČŌĪŖn%µÄŹĒ”””””””””£
A.4mol A£«2mol B
B.6mol C£«1mol D
C.1mol A£«0.5mol B£«1.5mol C£«0.5mol D
D.2mol A£«1mol B£«3mol C£«1mol D
E.2mol A£«1mol B£«3mol C£«2mol D
(2)ČōĪ¬³ÖøĆĢāĢõ¼ž²»±ä£¬½ö“ÓÉś³ÉĪļæŖŹ¼Åä±Č£¬ŅŖĒó“ļµ½Ę½ŗāŹ±£¬CµÄĪļÖŹµÄĮæČŌĪŖWmol£¬ŌņDµÄĘšŹ¼ĪļÖŹµÄĮæn(D)Ó¦Āś×ćµÄĢõ¼žŹĒ””””””””””””””(ÓĆŗ¬WµÄ“śŹżŹ½±ķŹ¾)”£
(3)“Ė·“Ó¦µÄv£tĶ¼ĻóČē¼×Ķ¼£¬ČōĘäĖūĢõ¼ž¶¼²»±ä£¬Ö»ŹĒŌŚ·“Ó¦Ē°¼ÓČėŗĻŹŹµÄ“߻ƼĮ£¬ŌņĘäv£tĶ¼ĻóČēŅŅĶ¼£¬ĒėÓĆ”°£½”¢£¼”¢£¾”±ĢīæÕ£ŗ
¢Ła1””””””a2£»¢Śb1””””””b2£»¢ŪĮ½Ķ¼ÖŠŅõÓ°²æ·ÖĆ껿£ŗ¼×””””””ŅŅ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģ½ĖÕŹ”ø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌĢā£ØĄķ£© ĢāŠĶ£ŗĢīæÕĢā
ŌŚŅ»øöČŻ»ż¹Ģ¶Ø²»±äµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦£ŗ2A(g)+B(g)==3C(g)+D(s)£¬ŅŃÖŖ½«2molAŗĶ1molB³äČėøĆČŻĘ÷ÖŠ£¬·“Ó¦ŌŚÄ³ĪĀ¶ČĻĀ“ļµ½Ę½ŗāŹ±£¬CµÄĪļÖŹµÄĮæĪŖWmol£¬CŌŚĘ½ŗā»ģŗĻĘųĢåÖŠµÄĢå»ż·ÖŹżĪŖn%”£
¢ÅæÉČĻ¶ØÉĻŹöæÉÄę·“Ó¦ŌŚŅ»¶ØĢõ¼žĻĀŅŃ“ļµ½»ÆŃ§Ę½ŗāדĢ¬µÄŹĒ (Ń”ĢīŠņŗÅ)”£
A”¢ĢåĻµŃ¹Ēæ²»ŌŁ±ä»Æ B”¢vÕż(A)£½2vÄę(B)
C”¢ĢåĻµµÄĆÜ¶Č²»ŌŁ±ä»Æ D”¢»ģŗĻĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ²»ŌŁ±ä»Æ
¢ĘČē¹ūÓĆĻĀĮŠĒéæöµÄÅä±Č×÷ĘšŹ¼Īļ£¬ŌŚĶ¬ŃłµÄČŻĘ÷ŗĶĪĀ¶ČĻĀ“ļµ½Ę½ŗā£¬Ęä½į¹ūŅ»¶ØŹĒ£ŗ
CµÄĪļÖŹµÄĮæĪŖ2Wmol£¬CŌŚĘ½ŗā»ģŗĻĘųĢåÖŠµÄĢå»ż·ÖŹżČŌĪŖn%µÄŹĒ
AӢ4molA+2molB BӢ6molC+1molD
CӢ1molA+0.5molB+1.5molC+0.5molD DӢ2molA+1molB+3molC+1molD
EӢ2molA+1molB+3molC+2molD
¢ĒČōĪ¬³ÖøĆĢāĢõ¼ž²»±ä£¬½ö“ÓÉś³ÉĪļæŖŹ¼Åä±Č£¬ŅŖĒó“ļµ½Ę½ŗāŹ±£¬CµÄĪļÖŹµÄĮæČŌĪŖWmol£¬ŌņDµÄĘšŹ¼ĪļÖŹµÄĮæn(D)Ó¦Āś×ćµÄĢõ¼žŹĒ £ØÓĆŗ¬WµÄ“śŹżŹ½±ķŹ¾£©
¢Č“Ė·“Ó¦µÄv”ŖtĶ¼ĻóČē¼×Ķ¼£¬ČōĘäĖūĢõ¼ž¶¼²»±ä£¬Ö»ŹĒŌŚ·“Ó¦Ē°¼ÓČėŗĻŹŹµÄ“߻ƼĮ£¬Ōņ
Ęäv”ŖtĶ¼ĻóČēŅŅĶ¼£¬ĒėÓĆ”°=”¢£¼”¢£¾”±ĢīæÕ£ŗ¢Ła1 a2£»¢Śb1 b2£»¢ŪĮ½Ķ¼ÖŠ
ŅõÓ°²æ·ÖĆ껿£ŗ¼× ŅŅ”£

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com