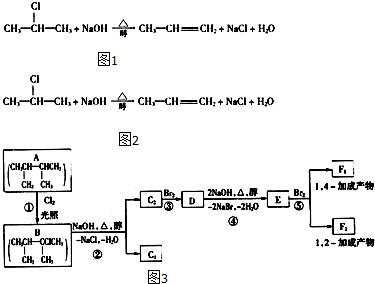
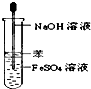
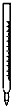��������1����ˮ����Ա�ʾ�ɣ�C
57H
106O
6+3H
2O��C
3H
8O
3�����ͣ�+A+2X��A����Է�������Ϊ280��ԭ�Ӹ�����ΪC��H��O=9��16��1����A�ķ���ʽΪC
9nH
16nO
n��n=
=2������A�ķ���ʽΪ��C
18H
32O
2������ԭ���غ�֪����ֱ������֬����ķ���ʽΪ��C
18H
36O
2���ṹ��ʽΪ��CH
3-��CH
2��
16-COOH��
��2��������ɷ�Ӧԭ������Ӧ�����ж�B��C�Ľṹ��ʽ��
��3���л������к�C��54.8%��H��5.58%��N��7.11%�����ຬO����O����������Ϊ1-54.8%-5.58%-7.11=32.51%�������л�����N��C����N��H����N��O����N��N��=
��
��
��
��9��11��4��1���������ʽΪC
9H
11NO
4��ʽ��Ϊ9��12+11+14+4��16=197��200���������⣬���ҷ���ʽΪC
9H
11NO
4��
��4�����Ǧ�-�����ᣬ�����в����ڼ���-CH
3��������FeCl
3��Һ������ɫ��Ӧ��˵�����з��ǻ���1mol A�������3molNaOH��ȫ��Ӧ��˵�������г����а������Ȼ�֮��Ӧ����2�����ǻ���
��5���ݡ����ȼ��ij�ǣ����ĵ����������ɵ�CO
2��H
2O�����ʵ�������ȡ��ɵó�ʵ��ʽΪCH
2O��
��6�����������Ϣ�ж��京�еĹ��������༰��Ŀ��Ȼ��ȷ����Ľṹ��ʽ�����д������������Һ��Ӧ�Ļ�ѧ����ʽ��
���
�⣺��1����ˮ����Ա�ʾ�ɣ�C
57H
106O
6+3H
2O��C
3H
8O
3�����ͣ�+A+2X��A����Է�������Ϊ280��ԭ�Ӹ�����ΪC��H��O=9��16��1����A�ķ���ʽΪC
9nH
16nO
n��n=
=2������A�ķ���ʽΪ��C
18H
32O
2������ԭ���غ�֪����ֱ������֬����ķ���ʽΪ��C
18H
36O
2���ṹ��ʽΪ��CH
3-��CH
2��
16-COOH��
�ʴ�Ϊ��C
18H
32O
2��
��2����B��C�Ļ���������KMnO
4��Һ�����ữ�õ��������ֲ��HOOC-��CH
2��
10-COOH��CH
3-��CH
2��
7-COOH��HOOC-��CH
2��
7-COOH��CH
3-��CH
2��
4-COOH�����ݷ�Ӧԭ����RCH=CHR��
RCOOH+R��COOH����B��C��Ϊͬ���칹���֪��B��C�к��е�Cԭ����һ����ȣ�����Խ��ֽ������ࣺHOOC-��CH
2��
10-COOH��CH
3-��CH
2��
4-COOH��CH
3-��CH
2��
7-COOH��HOOC-��CH
2��
7-COOH�ֱ�ΪB��C�е�һ�������Ը�����ط�Ӧ���ɵIJ����B��C�Ľṹ��ʽΪ��CH
3��CH
2��
7-CH=CH-��CH
2��
7-COOH��CH
3��CH
2��
4-CH=CH-��CH
2��
10-COOH��
�ʴ�Ϊ��CH
3��CH
2��
7-CH=CH-��CH
2��
7-COOH��CH
3��CH
2��
4-CH=CH-��CH
2��
10-COOH��
��3��ij�л���A��C��54.8%��H��5.58%��N��7.11%�����ຬO����O����������Ϊ1-54.8%-5.58%-7.11=32.51%�������л�����N��C����N��H����N��O����N��N��=
��
��
��
��9��11��4��1�������ʽΪC
9H
11NO
4��ʽ��Ϊ9��12+11+14+4��16=197��200���������⣬���ҵķ���ʽΪ��C
9H
11NO
4����Է�������Ϊ197��
�ʴ�Ϊ��197��
��4�����Ǧ�-�����ᣬ�����в����ڼ���-CH
3��������FeCl
3��Һ������ɫ��Ӧ��˵�����з��ǻ���1mol ���������3molNaOH��ȫ��Ӧ��˵�������г����а������Ȼ�֮��Ӧ����2�����ǻ������ܵĽṹ��
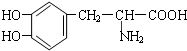
�ȣ���Ӧ��ͬ���칹����Ҫ�����ڱ������ǻ���λ�ã����ǻ�����ʱ����2��ͬ���칹�壬���ǻ����ʱ����3��ͬ���칹�壬���ǻ����ʱ����һ�ֽṹ������6��ͬ���칹�壬
�ʴ�Ϊ��
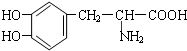
��
��5����Ϊ����ȼ�����ĵ����������ɵĶ�����̼��ˮ�����ʵ�������ȣ�
���Ը��Ƿ�����C��H��O����ԭ�����ʵ���֮��Ϊ��1��2����2+1-2��=1��2��1���ʸ��ǵ����ʽΪ��CH
2O��
�ʴ�Ϊ��CH
2O��
��6��������Է��������������ʽ��Է���������5�������ķ���ʽΪ����CH
2O��
5=C
5H
10O
5��
0.1mol�����ܻ�ԭ21.6g�����������ʵ���Ϊ��
=0.2mol�����л����к���1��ȩ����
0.1mol��������24g���ᷢ��������Ӧ��24g��������ʵ���Ϊ��
=0.4mol������к���4���ǻ���
������ֱ�����ӣ�����Ľṹ��ʽΪ��CH
2OH-CHOH-CHOH-CHOH-CHO������������Һ��Ӧ�Ļ�ѧ����ʽΪ��CH
2OH-CHOH-CHOH-CHOH-CHO+2Ag��NH
3��
2OH
CH
2OH-CHOH-CHOH-CHOH-COONH
4++2Ag��+3NH
3+H
2O��
�ʴ�Ϊ��CH
2OH-CHOH-CHOH-CHOH-CHO+2Ag��NH
3��
2OH
CH
2OH-CHOH-CHOH-CHOH-COONH
4++2Ag��+3NH
3+H
2O��

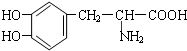 �ȣ���Ӧ��ͬ���칹����Ҫ�����ڱ������ǻ���λ�ã����ǻ�����ʱ����2��ͬ���칹�壬���ǻ����ʱ����3��ͬ���칹�壬���ǻ����ʱ����һ�ֽṹ������6��ͬ���칹�壬
�ȣ���Ӧ��ͬ���칹����Ҫ�����ڱ������ǻ���λ�ã����ǻ�����ʱ����2��ͬ���칹�壬���ǻ����ʱ����3��ͬ���칹�壬���ǻ����ʱ����һ�ֽṹ������6��ͬ���칹�壬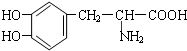 ��
��

 ��У����ϵ�д�
��У����ϵ�д�
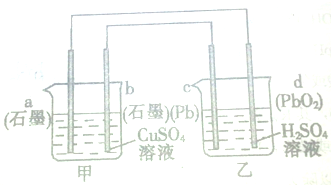
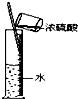
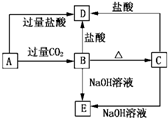 A��B��C��D��E���ֻ����������ij�ֳ���Ԫ�أ����ǵ�ת����ϵ��ͼ��ʾ������AΪ������Һ������ɫ��Ӧ��dz��ɫ������ɫ�ܲ�������BΪ���ܵİ�ɫ���壬A��E����������ͬ����ش��������⣺
A��B��C��D��E���ֻ����������ij�ֳ���Ԫ�أ����ǵ�ת����ϵ��ͼ��ʾ������AΪ������Һ������ɫ��Ӧ��dz��ɫ������ɫ�ܲ�������BΪ���ܵİ�ɫ���壬A��E����������ͬ����ش��������⣺