
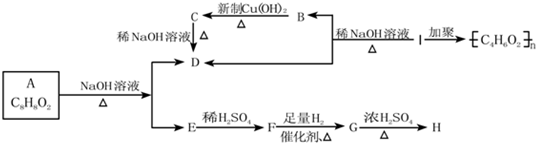
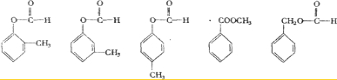
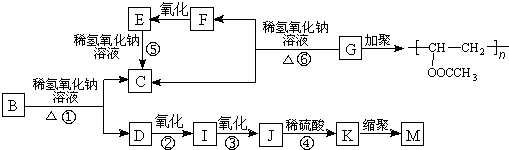
��֪�����ǻ���˫��̼ԭ��������ʱ����������ת������ѧʽΪC8H8O2���л��������µ�ת����ϵ��
����F����FeCl3��Һ����ɫ��IΪһ�ֲ���������
�ش�
��1���ṹ��ʽE ��B ��I ��
��2��д����Ӧ���ͣ�B��C �� G��H ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ
��A��D+E ��
��B��C ��
��4��д����A������ͬ�����š������ڷ����廯�����ͬ���칹��Ľṹ��ʽ(����A)�� �� �� ��
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
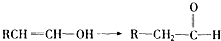


| �� |
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


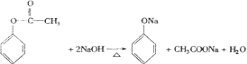
 +3NaOH
+3NaOH| ˮ |
 +NaBr+H2O
+NaBr+H2O +3NaOH
+3NaOH| ˮ |
 +NaBr+H2O
+NaBr+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]()
��ѧʽΪC8H8O2���л��������µ�ת����ϵ��

����F����FeCl3��Һ����ɫ��
�ش�
��1���ṹ��ʽ��E______________��B______________��I______________��
��2��д����Ӧ����
B![]() C��_________________________________________________________��
C��_________________________________________________________��
G![]() H��_________________________________________________________��
H��_________________________________________________________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ
��A![]() D+E��______________________________________________________��
D+E��______________________________________________________��
��B![]() C��________________________________________________________��
C��________________________________________________________��
��4��д����A������ͬ�Ĺ����ŵ�A�ķ����廯�����ͬ���칹��Ľṹ��ʽ������A����__________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣����Ϫ��ͩ��������ѧ�߶���ѧ����ĩ���Ի�ѧ������������ ���ͣ������
��10�֣���֪�����ǻ���˫��̼ԭ������ʱ����������ת��
��ѧʽΪC9H10O2���л���A�����µ�ת����ϵ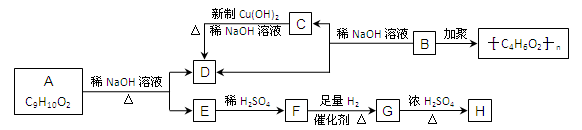
����F��FeCl3��Һ�ܷ�����ɫ��Ӧ����G��H�ķ�Ӧ�У��л���Hֻ��һ�ֽṹ����ʹ��
ˮ��ɫ���Իش��������⣺
��1��д���л���Ľṹ��ʽ��F ��H ��B ��
��2��д��A��E��D�Ļ�ѧ����ʽ�� ��
��3��д��C��D�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡΫ�������и���4���¿����ۻ�ѧ�Ծ��������棩 ���ͣ������
�л���A�ķ���ʽΪC9H10O2��A�ڹ������������ɵ�һ�����B���ɷ�������ת����ϵ(��������)��
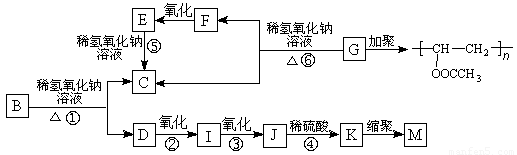
����K�������Ȼ�����Һ������ɫ��Ӧ���һ��ϵ�һԪȡ����ֻ�����ֽṹ��
��֪�������ǻ���˫��̼ԭ������ʱ����������ת����RCH��CHOH��RCH2CHO��
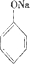
����ONa���������ϲ��ᱻ������
��ش��������⣺
��1��F��I�о�����ͬ�Ĺ����ţ��ù����ŵ�������??????????????? ��
��2�������仯������ˮ�ⷴӦ����???????????????????? ���Ӧ��ţ���
��3��д���ṹ��ʽ��G��??????????????????? ��M��????????????????????????? ��
��4��д����Ӧ���Ļ�ѧ����ʽ��???????????????????????????????????????????????????????? ��
��5����дһ����������Ҫ���A��ͬ���칹��?????????????????????????????? ��
I�����б���???? II���ܷ���������Ӧ������ʹ�Ȼ�����Һ����ɫ
III�������ϵ�һԪȡ����ֻ��һ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com