
ʵ�����Ʊ������������������ʵ�鲽�����£�ȡ�����ྻ����м������20%����30%��ϡ������Һ����50�桫80��ˮԡ�м��������ٲ������ݡ�����Һ���ȹ��ˣ���Һ�����Թ��У������������Թܿڡ����á���ȴһ��ʱ����ռ���Ʒ��
��1��д����ʵ���Ʊ����������Ļ�ѧ����ʽ��____________________________________��
��2��������Һ��ϡ�ᵼ��______________________________________________________��
��3������ˮԡ���ȵ�ԭ����____________________________________________________��
��4����Ӧʱ��м������Ŀ���ǣ������ӷ���ʽ��ʾ��______________________________��
��5����Һ���ȹ��˵�ԭ����____________________________________________________��
�����Թܿڵ�Ŀ����______________________________________________________��
��6��������ȴһ��ʱ������Թ��й۲쵽��������______________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2008�����������ۣ�28����ѧʵ������ȡ�Ȼ�������ķ���֮һ�ǽ�Ũ�������Ũ�����С������ͼ����ѡ�����������ڷ����ڻ����ø÷����Ʊ����ռ������Ȼ��������װ�ü�ͼ������ͼ�б��������Լ������������ظ�ʹ�ã��̶�װ�ò��ػ�������
��ʵ�����Ʊ������������������ʵ�鲽�����£�ȡ�����ྻ����м������20%��30%��ϡ������Һ����50�棭80��ˮԡ�м��������ٲ������ݡ�����Һ���ȹ��ˣ���Һ�����Թ��У������������Թܿڣ����á���ȴһ��ʱ����ռ���Ʒ��
��1��д����ʵ���Ʊ����������Ļ�ѧ����ʽ���ߣ� �ߣߡ�
��2��������Һ��ϡ�ᵼ�£ߣ� �ߣߡ�
��3������ˮԡ���ȵ�ԭ���ǣߣ� �ߣߡ�
��4����Ӧʱ��м������Ŀ���ǣ������ӷ���ʽ��ʾ���ߣ� �ߣߡ�
��5����Һ���ȹ��˵�ԭ���ǣߣ� �ߡ������Թܿڵ�Ŀ���ǣߣߣ� �ߡ�
��6��������ȴһ��ʱ������Թ��й۲쵽�������ǣ� �ߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008����ͨ�ߵ�ѧУ����ͳһ�����������⻯ѧ���֣������� ���ͣ�ʵ����
��2008�����������ۣ�28����ѧʵ������ȡ�Ȼ�������ķ���֮һ�ǽ�Ũ�������Ũ�����С������ͼ����ѡ�����������ڷ����ڻ����ø÷����Ʊ����ռ������Ȼ��������װ�ü�ͼ������ͼ�б��������Լ������������ظ�ʹ�ã��̶�װ�ò��ػ�������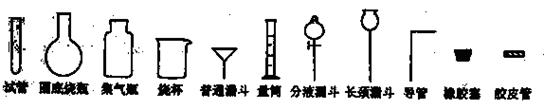
| |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�������и�����ѧ�ڵڶ����¿���ѧ���� ���ͣ�ʵ����
��9�֣���ѧʵ������ȡ�Ȼ�������ķ���֮һ�ǽ�Ũ�������Ũ�����С�
��1����2�֣������ͼ����ѡ�����������ڷ����ڻ����ø÷����Ʊ����ռ������Ȼ������塢β��������װ�ü�ͼ������ͼ�б��������Լ������������ظ�ʹ�ã��̶�װ�ò��ػ��������������ҳ��
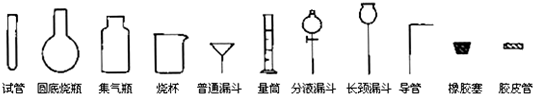
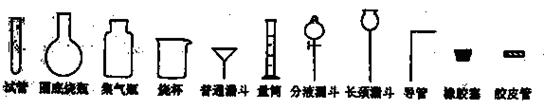
�Թ�Բ����ƿ����ƿ�ձ���ͨ©����Ͳ��Һ©������©������������Ƥ��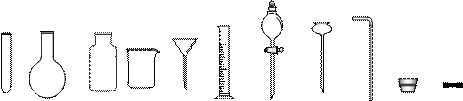
Դ:ѧ����ZXXK]
��2����(7��)ʵ�����Ʊ������������������ʵ�鲽�����£�ȡ�����ྻ����м������20%��30%��ϡ������Һ����50�棭80��ˮԡ�м��������ٲ������ݡ�����Һ���ȹ��ˣ���Һ�����Թ��У������������Թܿڣ����á���ȴһ��ʱ����ռ���Ʒ��
��д����ʵ���Ʊ����������Ļ�ѧ����ʽ����
�ڲ���ˮԡ���ȵ�ԭ���ǡ�
��������Һ��ϡ�ᵼ������
�ܷ�Ӧʱ��м������Ŀ���ǣ������ӷ���ʽ��ʾ����
����Һ���ȹ��˵�ԭ���ǡ������Թܿڵ�Ŀ���ǡ�
������ȴһ��ʱ������Թ��й۲쵽�������ǡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ������ѧ�ڵڶ����¿���ѧ���� ���ͣ�ѡ����
��9�֣���ѧʵ������ȡ�Ȼ�������ķ���֮һ�ǽ�Ũ�������Ũ�����С�
��1����2�֣������ͼ����ѡ�����������ڷ����ڻ����ø÷����Ʊ����ռ������Ȼ������塢β��������װ�ü�ͼ������ͼ�б��������Լ������������ظ�ʹ�ã��̶�װ�ò��ػ��������������ҳ��[��Դ:Z#xx#k.Com]
�Թ�Բ����ƿ����ƿ�ձ���ͨ©����Ͳ��Һ©������©������������Ƥ��
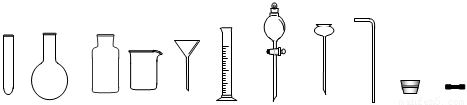
��2����(7��)ʵ�����Ʊ������������������ʵ�鲽�����£�ȡ�����ྻ����м������20%��30%��ϡ������Һ����50�棭80��ˮԡ�м��������ٲ������ݡ�����Һ���ȹ��ˣ���Һ�����Թ��У������������Թܿڣ����á���ȴһ��ʱ����ռ���Ʒ��
��д����ʵ���Ʊ����������Ļ�ѧ����ʽ����
�ڲ���ˮԡ���ȵ�ԭ���ǡ�
��������Һ��ϡ�ᵼ������
�ܷ�Ӧʱ��м������Ŀ���ǣ������ӷ���ʽ��ʾ����
����Һ���ȹ��˵�ԭ���ǡ������Թܿڵ�Ŀ���ǡ�
������ȴһ��ʱ������Թ��й۲쵽�������ǡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com