
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| n |
| V |
| 0.2mol |
| 2L |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013Ń§ÄźÉ½¶«Ź”¼ĆÄĻŹŠøßČż4ŌĀ¹®¹ĢŠŌѵĮ·Ąķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ¼ĘĖćĢā
ĮņŌŖĖŲµÄ»ÆŗĻĪļŌŚÉś²ś”¢Éś»ī֊ӊ׏ć·ŗµÄÓ¦ÓĆ”£
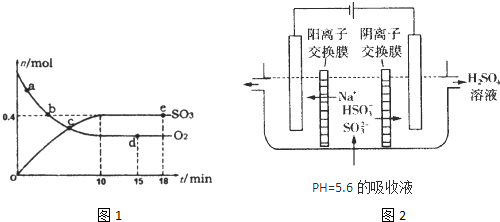
£Ø1£©400”ę£¬1.01”Į PaĻĀ£¬ČŻ»żĪŖ1£®0LµÄĆܱÕČŻĘ÷ÖŠ³äČė0.5molSO2,
(g)ŗĶ0.3 molO2 (g)£¬·¢Éś·“Ó¦2SO2(g)£«O2(g)
PaĻĀ£¬ČŻ»żĪŖ1£®0LµÄĆܱÕČŻĘ÷ÖŠ³äČė0.5molSO2,
(g)ŗĶ0.3 molO2 (g)£¬·¢Éś·“Ó¦2SO2(g)£«O2(g) 2SO3(g)
”÷H£½£198kJ/mol”£·“Ó¦ÖŠn(SO3)ŗĶn(O2)Ėꏱ¼ä±ä»ÆµÄ¹ŲĻµČēÓŅĶ¼ĖłŹ¾
2SO3(g)
”÷H£½£198kJ/mol”£·“Ó¦ÖŠn(SO3)ŗĶn(O2)Ėꏱ¼ä±ä»ÆµÄ¹ŲĻµČēÓŅĶ¼ĖłŹ¾ ”£·“Ó¦µÄĘ½ŗā³£ŹżK£½_______£»0µ½10 minÄŚÓĆSO2±ķŹ¾µÄĘ½¾ł·“Ó¦ĖŁĀŹ_________”£øł¾ŻĶ¼ÖŠŠÅĻ¢£¬ÅŠ¶ĻĻĀĮŠŠšŹöÖŠÕżČ·µÄŹĒ_____£ØĢīŠņŗÅ£©”£
”£·“Ó¦µÄĘ½ŗā³£ŹżK£½_______£»0µ½10 minÄŚÓĆSO2±ķŹ¾µÄĘ½¾ł·“Ó¦ĖŁĀŹ_________”£øł¾ŻĶ¼ÖŠŠÅĻ¢£¬ÅŠ¶ĻĻĀĮŠŠšŹöÖŠÕżČ·µÄŹĒ_____£ØĢīŠņŗÅ£©”£
A£®aµćŹ±æĢµÄÕż·“Ó¦ĖŁĀŹ±ČbµćŹ±æĢµÄ“ó
B£®cµćŹ±æĢ·“Ó¦“ļµ½Ę½ŗāדĢ¬
C£®dµćŗĶeµćŹ±æĢµÄc(O2)ĻąĶ¬
D£®Čō5 00”ę£¬1.01”Į105PaĻĀ£¬·“Ó¦“ļµ½Ę½ŗāŹ±£¬n( SO3) ±ČĶ¼ÖŠeµćŹ±æĢµÄÖµ“ó
£Ø2£©ÓĆNaOHČÜŅŗĪüŹÕ¹¤Ņµ·ĻĘųÖŠµÄSO2£¬µ±ĪüŹÕŅŗŹ§Č„ĪüŹÕÄÜĮ¦Ź±£¬25”ꏱ²āµĆČÜŅŗµÄpH=5.6£¬ČÜŅŗÖŠNa£«,H£«, HSO3£,SO32£Ąė×ÓµÄÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ__________________”£
£Ø3£©æÉĶعżµē½ā·ØŹ¹£Ø2£©ÖŠµÄĪüŹÕŅŗŌŁÉś¶ųŃ»·ĄūÓĆ£Øµē¼«¾łĪŖŹÆÄ«µē¼«£©£¬Ę乤×÷Ź¾ŅāĶ¼ČēĻĀ£ŗ

HSO3£ŌŚŃō¼«ŹŅ·“Ó¦µÄµē¼«·“Ó¦Ź½ĪŖ________________________£¬Ņõ¼«ŹŅµÄ²śĪļ_________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com