
| n |
| V |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
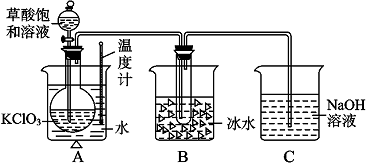
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ�����������Թ��У������������0.1mol?L-1 CH3COOH�� | |
| ����2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
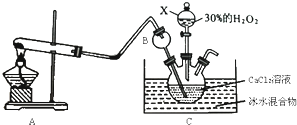
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
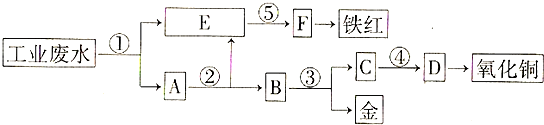
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
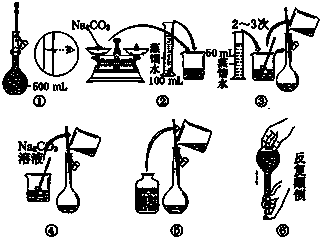 ��1����ijҩƷ����ԼΪ32g
��1����ijҩƷ����ԼΪ32g| 50g | 20g | 20g | 10g | 5g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | K+��Ag+��Mg2+��Ba2+ |
| ������ | NO3-��CO32-��SiO32-��SO42- |
| ��� | ʵ������ | ʵ���� |
| �� | �����Һ�� ��������ϡ���� | ������ɫ�������ų���״����0.56 L���� |
| �� | ����ķ�Ӧ���Һ���ˣ��Գ���ϴ�ӡ����������أ��������ù������� | ��������Ϊ2.4 g |
| �� | ������Һ�еμ�BaCl2��Һ | ���������� |
| ������ | NO3- | CO32-�� | SiO32- | SO42- |
| c/mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
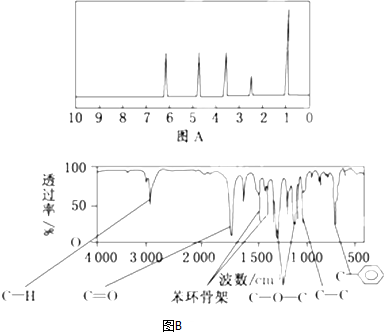
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� | ���볣����Ka�� | �� | ���볣����Ka�� |
| CH3COOH | 1.8��10-5 | H3PO4 | K1=7.11��10-3 |
| H3PO3 | K1=3.7��10-2 | K2=6.23��10-8 | |
| K2=2.9��10-7 | K3=4.5��10-13 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1�����ʯ��ʯī��Ϊ̼��ͬ�������壬��������������ʱȼ������һ����̼������������ʱ���ȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ��
��1�����ʯ��ʯī��Ϊ̼��ͬ�������壬��������������ʱȼ������һ����̼������������ʱ���ȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ��| ��ѧ�� | N-H | N-N | O�TO | N��N | O-H |
| ���ܣ�kJ/mol�� | 386 | 167 | 498 | 946 | 460 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com