
A��B��X��Y��Z��ԭ���������ε����Ķ�����Ԫ�أ�����A��Yͬ���壬X��Zͬ���壬A��B��X��X�����γ�10�����ӻ����B��Z������������֮��2��3������������Y2X2��ˮ��Ӧ����X�ĵ��ʣ�����Һ��ʹ��̪��Һ��졣��ش��������⡣
��1��Z��ԭ�ӽṹʾ��ͼΪ ��������BA4�ĵ���ʽΪ ��
��2��������Y2X2�к��еĻ�ѧ�������� ������ţ���
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
��3��������A2X��A2Z�У��е�ϸߵ��� ���ѧʽ��������Ҫԭ���� ��
��4��A��X��A��Z�����γ�18�����ӵĻ���������ֻ��������Ӧ�Ļ�ѧ����ʽΪ ��
��5�����³�ѹ�£���A��B��X����ɵ�Һ̬���ʼס�����2.3g����������X�ĵ��ʳ�ַ�Ӧ�����ɱ�״����2.24L��BX2�����2.7g��A2XҺ�壬ͬʱ�ų�68.35kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
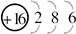
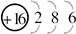
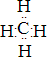
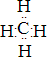
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
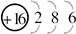
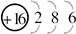
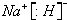
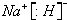
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


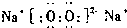
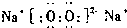
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015���½���³ľ���и�һ��ƽ�аࣩ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
A��B��X��Y��Z��ԭ���������ε����Ķ�����Ԫ�أ�����A��Yͬ���壬X��Zͬ���壬A��B��A��X�����γ�10�����ӻ����B��Z������������֮��Ϊ2�U3������������Y2X2��ˮ��Ӧ����X�ĵ��ʣ�����Һ��ʹ��̪��Һ��졣��ش��������⡣
(1) X�����ڱ��е�λ����__________________________
(2) ������Y2X2�ĵ���ʽΪ �������еĻ�ѧ�������� ������ţ���
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
(3) A��X��A��Z�����γ�18�����ӵĻ���������ֻ��������Ӧ����Z�Ļ�ѧ����ʽΪ_____________________________________��
(4) A�ĵ�����X�ĵ��ʿ��Ƴ����͵Ļ�ѧ��Դ��KOH��Һ���������Һ���������缫���ɶ����̼�Ƴɣ�ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ磬�����������缫��ӦʽΪ_________________________��________________________��
(5) д��������Y2X2��ˮ��Ӧ�����ӷ���ʽ_____________________��
(6) B�����������ĽṹʽΪ_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ĵ�ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��12�֣�A��B��X��Y��Z��ԭ���������ε����Ķ�����Ԫ�أ�����A��Yͬ���壬X��Zͬ���壬A��B��X�����γ�10�����ӻ����B��Z������������֮��Ϊ2��3������������Y2X2��ˮ��Ӧ����X�ĵ��ʣ�����Һ��ʹ��̪��Һ��죮��ش��������⣮
(1)Z�����ӽṹʾ��ͼΪ ��������BA4�ĵ���ʽΪ ��
(2)������Y2X2�к��еĻ�ѧ��������________ (�����)��
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
(3)������A2X��A2Z�У��е�ϸߵ���_______ (�ѧʽ)������Ҫԭ��_______ _______ _______ ��
(4)A��X��A��Z�����γ�18�����ӵĻ���������ֻ��������Ӧ����A2X��Z���ʣ���д����ѧ����ʽ____________________ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com