
 Na2S4O6+2NaI���������Ŵ����Һ��I��Ũ�ȷŴ�Ϊԭ��Һ��I��Ũ�ȵģ���ǰ����Һ�����ȣ�
Na2S4O6+2NaI���������Ŵ����Һ��I��Ũ�ȷŴ�Ϊԭ��Һ��I��Ũ�ȵģ���ǰ����Һ�����ȣ�| A��2�� | B��4�� | C��36�� | D��6�� |


 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����100mL��Ͳ��ȡ5.2mL��ϡ���� |
| B����500mL����ƿ����500mL0.2mol /L��NaOH��Һ |
| C����������ƽ����11.75gNaCl���� |
| D���÷�Һ©�����й����Һ��ķ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ����Ϊ98%���ܶ�Ϊ1.84g/�M3��Ũ����______ mL����С�������1λ��Ч���֣�
����Ϊ98%���ܶ�Ϊ1.84g/�M3��Ũ����______ mL����С�������1λ��Ч���֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ϲ��Ϻ�ɫ���²�ӽ���ɫ | B�����ȡ������Ϻ�ɫ |
| C�����ȡ�������ɫ | D���ϲ�ӽ���ɫ���²��Ϻ�ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ȡ��Na2CO3��Һʱ������ȡ�����࣬Ϊ�˲��˷ѣ��ѹ������Լ������Լ�ƿ�� |
| B��Ba(NO3)2 ������ˮ����˿ɽ�����Ba(NO3)2 �ķ�Һ����ˮ���У�����ˮ������ˮ�� |
| C������������ʹNaCl����Һ������ʱ��Ӧ����������NaCl��Һȫ���������� |
| D��ϡ��Ũ����ʱ��Ӧ�ý�Ũ���Ỻ��ע��ˮ�У������Ͻ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��Һ����������ش��������⣺
��Һ����������ش��������⣺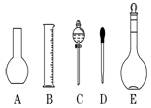
| A��������һ�����ȷŨ�ȵı���Һ | B���ɳ���������Һ |
| C�������������ܽ�������� | D��ʹ��ǰ�������Ƿ�©ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ŨH2SO4������500 mL 0.2
ŨH2SO4������500 mL 0.2 mol /L��ϡH2SO4�������������ٲ���������Ͳ���ձ��ܽ�ͷ�ι�
mol /L��ϡH2SO4�������������ٲ���������Ͳ���ձ��ܽ�ͷ�ι� Ϊ���¼������裺�ٳ����ڼ�����ܽ��ҡ�Ȣ�ת�Ƣ�ϴ�Ӣ߶��ݢ���ȴ������ȷ�IJ���˳���� ��
Ϊ���¼������裺�ٳ����ڼ�����ܽ��ҡ�Ȣ�ת�Ƣ�ϴ�Ӣ߶��ݢ���ȴ������ȷ�IJ���˳���� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 00 mL����
00 mL���� �Ƶ�����ƿ֮ǰ��Ҫ�ָ�����������Ϊ_______________________________
�Ƶ�����ƿ֮ǰ��Ҫ�ָ�����������Ϊ_______________________________ _��2�֣���
_��2�֣��� .����ʱ������ˮ���������˿̶�_______________
.����ʱ������ˮ���������˿̶�_______________�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
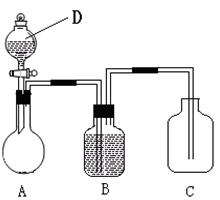
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com