
��10�֣�ʵ�����мס�����ƿ��ɫ��Һ������һƿ��ϡ���ᣬ��һƿ��̼������Һ��Ϊ�ⶨ�ס�����ƿ��Һ�ijɷּ����ʵ���Ũ�ȣ���������ʵ�飺
����ȡ25.00mL����Һ��������������Һ15.00mL�����ռ���224mL����״��������
����ȡ15.00mL����Һ�������������Һ25.00mL�����ռ���112mL����״�������塣
��1���жϣ����� ��Һ������ ��Һ��
��2��д��������������Ӧ�����ӷ���ʽ ��
��3������Һ�����ʵ���Ũ���Ƕ��٣�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
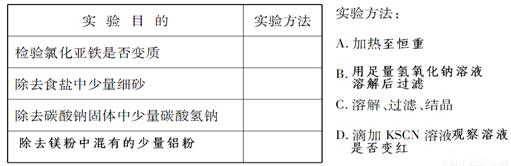
| ʵ��Ŀ�� | ���鷽�� |
| �����Ȼ������Ƿ���� | D D |
| ��ȥʳ��������ϸɳ | C C |
| ��ȥ̼���ƹ���������̼������ | A A |
| ��ȥþ���л��е��������� | B B |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣�
��1��ʵ�����мס�����ƿ��ɫ��Һ������һƿ��ϡ���ᣬ��һƿ��̼������Һ��Ϊ�ⶨ�ס�����ƿ��Һ�ijɷ֣���������ʵ�飺ȡ����Һ�������л�����������Һ�����ߵμӱ����۲쵽��ʼ�������������д����������ɡ�ʵ���������������Ӧ�����ӷ���ʽΪ�� ������ ��Һ������ ��Һ��
(2)Ϊ�˴ﵽ�±����е�ʵ��Ŀ�ģ���ѡ����ʵ�ʵ�鷽����������������Ӧ�Ŀո���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ʡĵ����һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��8�֣�
��1��ʵ�����мס�����ƿ��ɫ��Һ������һƿ��ϡ���ᣬ��һƿ��̼������Һ��Ϊ�ⶨ�ס�����ƿ��Һ�ijɷ֣���������ʵ�飺ȡ����Һ�������л�����������Һ�����ߵμӱ����۲쵽��ʼ�������������д����������ɡ�ʵ���������������Ӧ�����ӷ���ʽΪ�� ������ ��Һ������ ��Һ��
(2)Ϊ�˴ﵽ�±����е�ʵ��Ŀ�ģ���ѡ����ʵ�ʵ�鷽����������������Ӧ�Ŀո���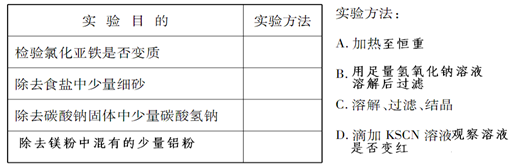
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��8�֣�
��1��ʵ�����мס�����ƿ��ɫ��Һ������һƿ��ϡ���ᣬ��һƿ��̼������Һ��Ϊ�ⶨ�ס�����ƿ��Һ�ijɷ֣���������ʵ�飺ȡ����Һ�������л�����������Һ�����ߵμӱ����۲쵽��ʼ�������������д����������ɡ�ʵ���������������Ӧ�����ӷ���ʽΪ�� ������ ��Һ������ ��Һ��
(2)Ϊ�˴ﵽ�±����е�ʵ��Ŀ�ģ���ѡ����ʵ�ʵ�鷽����������������Ӧ�Ŀո���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�����мס�����ƿ��ɫ��Һ��һƿ��ϡ���ᣬ��һƿ��NaAlO2
��Һ��Ϊ�ⶨ�ס�����ƿ��Һ�ijɷּ����ʵ���Ũ�ȣ���������ʵ�飺
�������Һ�л����μ�����Һ������������������������Һ�л����μӼ���Һ����ʼ��������һ��ʱ������������
��ȡ40mL����Һ�������л����������Һ20mL������������3.12g��
��1����ʢװ���� ��
��2���������Һ�л����μ�����Һ���������Ⱥ�����Ӧ�����ӷ���ʽΪ��
�� ��
��3�����г��������ʵ���Ϊ��__________mol������Һ�����ʵ���Ũ��Ϊ_____
mol/L������Һ�����ʵ���Ũ��Ϊ_________mol/L��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com