
ij��ɫ��Һ�к��У���Na+����Ba2+����C1������Br������SO![]() ����SO
����SO![]() �����е�һ�ֻ��֣����ν�������ʵ�飬��ÿ�������Լ����������۲쵽���������£�
�����е�һ�ֻ��֣����ν�������ʵ�飬��ÿ�������Լ����������۲쵽���������£�
| ���� | ���� | ���� |
| �� | ��pH��ֽ���� | ��Һ��pH����7 |
| �� | ����Һ�е�����ˮ���ټ���CC14������ | CC14��ʳ�ɫ |
| �� | ���������Һ�м���Ba(NO3)2��Һ��ϡHNO3 | �а�ɫ�������� |
| �� | ���ˣ�����Һ�м���AgNO3��Һ��ϡHNO3 | �а�ɫ�������� |
���н�����ȷ����
A���϶��������ӵĢ٢ܢ�
B���϶�û�е������Ǣڢ�
C�����ܺ��е������Ǣ٢ڢ�
D������ȷ���������Ǣ٢ۢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2011?ʯ��ɽ��һģ��ij��ɫ��Һ�к��У���Na+����Ba2+����Cl-����Br-����SO2-3����SO2-4-�����е�һ�ֻ��֣����ν�������ʵ�飬��ÿ�������Լ����������۲쵽���������£�
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
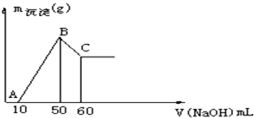 ij��ɫ��Һ�к���H+��Al3+��Mg2+���������ӣ���μ���0.1moL?L-1NaOH��Һ������NaOH��Һ�����X�ᣩ�����ɳ�����Y�ᣩ֮��ĺ�����ϵ��ͼ��ʾ������Һ��H+��Al3+��Mg2+���������ӵ����ʵ���Ũ��֮��Ϊ��������
ij��ɫ��Һ�к���H+��Al3+��Mg2+���������ӣ���μ���0.1moL?L-1NaOH��Һ������NaOH��Һ�����X�ᣩ�����ɳ�����Y�ᣩ֮��ĺ�����ϵ��ͼ��ʾ������Һ��H+��Al3+��Mg2+���������ӵ����ʵ���Ũ��֮��Ϊ���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2- 3 |
2- 4 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Pt˿���� |
| �� |
| +�Ȼ�����Һ |
| �� |
| +ϡHCl�ữ |
| �� |
| +ϡ��ˮ |
| �� |
| +�ռ���Һ |
| �� |
| ���� |
| �� |
| A������ɫ��Һ�п�����������Һ |
| B��������ʵ���У�ֻ�У�3�������ܺ����� |
| C������ܵ����ӷ���ʽΪ��Al3++3OH-=Al��OH��3�� |
| D������Ļ�ѧ����ʽΪ��NaAlO2+H2O+CO2=Al��OH��3��+NaHCO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com