
��ҵ�Ƽ�����ʳ��ˮ��ͨ�백���Ͷ�����̼�����̼�����ƾ��壬���ķ�Ӧԭ���������л�ѧ����ʽ��ʾ��
NH3��CO2��H2O��NH4HCO3������
NH4HCO3��NaCl(����)��HaHCO3����NH4Cl������
������̼�����ƾ�����ȷֽ�ɵõ����
��ش�
(1)��ҵ���ƴ����г������������Ȼ������ʣ���ԭ����________��
(2)���мס��ҡ�������С���ѧ�������ⶨij��ҵ������Ʒ��Na2CO3�������������ֱ���Ʋ����ʵ�����£�
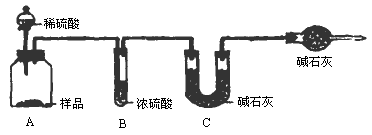
���飺ȡ10.00g��Ʒ��������ͼ��ʾװ�ã������Ӧ��װ��C�м�ʯ�ҵ�����Ϊ3.52g��
���飺ȡ10.00g��Ʒ�����1000mL��Һ���ü�ʽ�ζ�����ȡ25.00mL������ƿ�У����������ָʾ������0.150mol��L��1�ı�������Һ�ζ����յ�(�йط�ӦΪNa2CO3��2HCl��2NaCl��2H2O��CO2��)���������ƽ��ʵ����������������ƽ��ֵΪ30.00mL��
���飺ȡ10.00g��Ʒ�������м�����������ᣬֱ����Ʒ��������ð�����������������ﲢ�ڸ���������ȴ�����º�������������ȡ���ȴ��������ֱ���������Ĺ���������������Ϊֹ����ʱ���ù��������Ϊ10.99g�������������������

|
����(1)NaHCO3�ǴӺ����� ����(2)����,84.8��,ʧ�ܵ�ԭ�������ɵ�CO2û�б�ȫ�����գ���һ���������ڷ�Ӧ�����У����²ⶨ���ƫ�ͣ�;����,95.4��;����,95.4�� |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ����ׯ�ж�OO�߽������һ�ε��п��ԡ���ѧ���� ���ͣ�022
| |||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2006��2007ѧ�꽭���а�У������һ�ε��в��Ի�ѧ���⼰�� ���ͣ�058
| |||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫����2008�����5���¿���ѧ�Ծ� ���ͣ�058
��ҵ�ƴ������ʳ��ˮ��ͨ�백���Ͷ�����̼�����̼�����ƾ��壬���ķ�Ӧԭ���������л�ѧ����ʽ��ʾ��NH3��CO2��H2O��NH4HCO3������
NH4HCO3��NaCl(����)��NaHCO3����NH4Cl������
������̼�����ƾ�����ȷֽ�ɵõ����(������Ʒ��Na2CO3����������Ϊ92%~96%)��ش�
(1)��ҵ���ƴ����г������������Ȼ������ʣ���ԭ��________��
(2)���мס��ҡ�������С��ѧ�������ⶨij��ҵ������Ʒ��Na2CO3�������������ֱ���Ʋ����ʵ�����£�
���飺ȡ10.00 g��Ʒ��������ͼ��ʾװ�ã������Ӧ��װ��C�м�ʯ�ҵ�����Ϊ3.52 g��
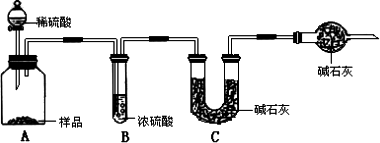
���飺ȡ10.00 g��Ʒ�����1000 mL��Һ���ü�ʽ�ζ�����ȡ25.00 mL������ƿ�У�����2�η�̪��ָʾ������0.150 mol��L��1�ı�������Һ�ζ����յ�(�йط�ӦΪNa2CO3��HCl��NaCl��NaHCO3)���������ƽ��ʵ����������������ƽ��ֵΪ15.00 mL��
���飺ȡ10.00 g��Ʒ�������м�����������ᣬֱ����Ʒ��������ð�����������������ﲢ�ڸ���������ȴ�����º�������������ȡ���ȴ��������ֱ���������Ĺ���������������Ϊֹ����ʱ���ù��������Ϊ10.99 g�������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����
 Na2CO3+CO2��+H2O��ij������ȤС��ͬѧ�����ա������Ƽ��ԭ�����������ͼ��ʾ��һ��ʵ��װ�ã�
Na2CO3+CO2��+H2O��ij������ȤС��ͬѧ�����ա������Ƽ��ԭ�����������ͼ��ʾ��һ��ʵ��װ�ã� 
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com