

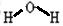 ��FΪNH3�������ĵ���ʽΪ��
��FΪNH3�������ĵ���ʽΪ��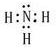 ����Ӧ����Ba��NO3��2+H2SO4��Ũ��=BaSO4��+2HNO3�����ڸ��ֽⷴӦ��
����Ӧ����Ba��NO3��2+H2SO4��Ũ��=BaSO4��+2HNO3�����ڸ��ֽⷴӦ��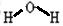 ��
��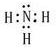 �����ֽⷴӦ��
�����ֽⷴӦ��
| ||
| ||


 ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��SO3+H2O=H2SO4 |
| B��2Na2O2+2H2O=4NaOH+O2�� |
| C��2F2+2H2O=4HF+O2 |
| D��Mg+2H2O=Mg��OH��2+H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CuSO4��Һ����H2S���壺Cu2++H2S��CuS��+2H+ | ||
B��Ca��HCO3��2��Һ��������NaOH��Һ��ϣ�Ca2++2HC
| ||
| C������������Һ�м����������ữ�Ĺ���������Һ��Fe2++2H++H2O2��Fe2++2H2O | ||
| D�����۵⻯����Һ�ڿ����б�����4I-+O2+2H2O��2I2+4OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ |
| B������lmol/L�Ĵ�����Һl00mL |
| C������������0.5mol/L������ |
| D������������lmol/L��NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��̼�����Ƶ�ˮ�ⷴӦ��HCO3-+H2O�TCO32-+H3O+ | ||||
| B����������Һ�еμӹ�����ˮ��Ag++2NH3?H2O=[Ag��NH3��2]++2H2O | ||||
C����ͭ���缫�������ͭ��Һ��2Cu2++2H2O
| ||||
| D��������[KAl��SO4��2]��Һ����μ���Ba��OH��2��Һ��SO42-ǡ����ȫ������2Al3++3SO42-+3Ba2++6OH-=2Al��OH��3��+3BaSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com