
A��B��C��D��E��Ԫ�����ڱ������ֶ�����Ԫ�أ�ԭ�������������� A��B��C��Dλ��ͬһ���ڡ���֪Aԭ�Ӻ�����3���ܼ�����ÿ���ܼ��ϵ����ɵĵ�����Ŀ��ͬ��C��Eͬ���壬��C�ĵ���Ϊ�����е���Ҫ�ɷݡ�XԪ�ص�ԭ�Ӻ�����26���˶�״̬��ȫ����ͬ�ĵ��ӡ��ش��������⣺
��1��C��D��E�е�һ������������ ����Ԫ�ط��ţ���X�ļ۵����Ų�ʽΪ
��
��2��B����̬�⻯����ӳ� �Ρ��÷��ӵ�����ԭ�ӵ��ӻ���ʽΪ ��
 ��3��A��һ�ֵ�����Է�������Ϊ720�����ӹ���Ϊһ��32����,
��3��A��һ�ֵ�����Է�������Ϊ720�����ӹ���Ϊһ��32����,
|
�ֵ����Цм�����ĿΪ ��
��4��XԪ�ض�Ӧ�ĵ������γɾ���ʱ��������ͼ2��ʾ
 �Ķѻ���ʽ�������ֶѻ�ģ�͵���λ��Ϊ ��
�Ķѻ���ʽ�������ֶѻ�ģ�͵���λ��Ϊ ��
���X��ԭ�Ӱ뾶Ϊa cm�������ӵ³�����ֵΪ
NA�������˵��ʵ��ܶȱ���ʽΪ g/cm3
|
 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��Ӧ��2A��B2C������A��B��C��Ϊ���壬��ͼ�е������Ǹ÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�����ߣ�x���ʾ�¶ȣ�y���ʾB��ת���ʣ�ͼ����a��b��c���㣬��ͼ��ʾ��������������ȷ���� (����)��
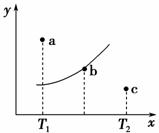
A���÷�Ӧ�Ƿ��ȷ�Ӧ
B��b��ʱ��������ƽ��Ħ���������ٱ仯
C��T1�¶���a���ʾ����ﵽƽ�⣬���Բ�ȡ����ѹǿ�ķ���
D��c��ɱ�ʾv(��)��v(��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
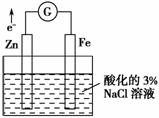
��ѡ���еĵ缫����Һ����ͼ��ʾװ�ÿ����ԭ��ء������������۵�������ȷ���� (����)��

| ѡ�� | �缫a | �缫b | A��Һ | B��Һ | �������� |
| A | Cu | Zn | CuSO4 | ZnSO4 | һ��ʱ���a���ӵ�������b���ٵ�������� |
| B | Cu | Zn | ϡ H2SO4 | ZnSO4 | ��������������b���ƶ� |
| C | Fe | C | NaCl | FeCl3 | ���·����ת�Ʒ���b��a |
| D | C | C | FeCl3 | KI������ ���Һ | ����ʼʱֻ����FeCl3��ҺŨ�ȣ�b��������Һ�������ٶȼӿ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ��ȤС��Ե绯ѧ���������ʵ��̽����
��.������ͼ��ʾװ��̽�������ķ�����ʩ��ʵ��������п�缫�����ܽ⣬���缫���������ݲ�����
(1)д�������ĵ缫��Ӧ________
________________________________________________________________��
(2)ijѧ����Ϊ�����缫���ܲ��뷴Ӧ�����Բ����������衣
���� 1 �������뷴Ӧ������������Fe2����
���� 2 �������뷴Ӧ������������Fe3����
���� 3 ��________________________________________________________��
(3)Ϊ��̽������1 ��2������ȡ���²�����
��ȡ0.01 mol��L��1 FeCl3��Һ 2 mL���Թ��У�����������ۣ�
��ȡ�������Թܵ��ϲ���Һ����2��K3[Fe(CN)6]��Һ��������ɫ������
��ȡ��������������Һ����2�� K3[Fe(CN)6]��Һ��δ����ɫ�������ɣ�
��ȡ��������������Һ����2�� KSCN ��Һ��δ����Һ��졣
�ݢڡ��ۡ�������ó��Ľ�����__________________ _____________________________________��
(4)��ʵ��ԭ����Ӧ���ڷ���������ʴ�����پ�һ������������ʴ�Ĵ�ʩ��_________________________________________________________________��
��.������ͼ��ʾװ������� 50 mL 0.5 mol��L��1�� CuCl2 ��Һʵ�顣

ʵ���¼��
A���������л���ɫ���������������ʹʪ��ĵ����⻯����ֽ�ȱ�������ɫ(��ʾ��Cl2 ��������ǿ��IO )��
)��
B�����һ��ʱ���Ժ����������������ͭ�⣬���������������ݺ�dz��ɫ���塣
(1)����ʵ���¼ A ����ֽ��ɫ�ı仯�������ӷ���ʽ���ͣ���________________��______________��
(2)����ʵ���¼ B ��dz��ɫ���������_____________________________
(д��ѧʽ)���Է������ɸ����ʵ�ԭ��________________________________
________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����ĵ���ƽ�ⳣ�����±�������ѡ����ȷ���ǣ� ��
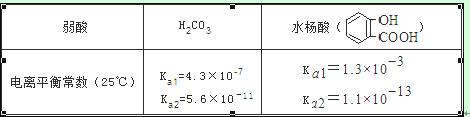
A�������£���Ũ�ȡ��������NaHCO3 ��ҺpHС�� ��ҺpH
��ҺpH
B�������£���Ũ�ȡ��������Na2 CO3 ��Һ�� 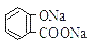 ��Һ��������������ǰ��С�ں���
��Һ��������������ǰ��С�ں���
C��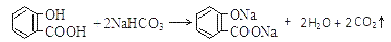
D��ˮ����ĵڶ������� Ka2 ԶС�ڵ�һ������Ka1 ��ԭ��֮һ�� ���γɷ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������������ȷ����(����)
A��1.2 g���ʯ�к��е�̼̼������Ϊ0.4NA
B��4.4 g������̼�к��еĹ��õ��Ӷ���Ϊ0.4NA
C������ʱ11.2 L��ϩ����������ȫȼ��ת�Ƶĵ�����Ϊ6.0NA
D��������0.1 mol��L��1�������Һ�У�NH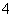 ��H������һ������0.1NA
��H������һ������0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ܹ�˵��һ�����淴ӦH2(g)��I2(g)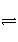
 2HI(g)�Ѵﵽƽ��״̬����(����)
2HI(g)�Ѵﵽƽ��״̬����(����)
A��1 mol H��H�����ѵ�ͬʱ��1 mol H��I���γ�
B��1 mol H��H�����ѵ�ͬʱ��2 mol H��I���γ�
C��1 mol I��I�����ѵ�ͬʱ��2 mol HI���γ�
D��1 mol H��H�����ѵ�ͬʱ��1 mol I��I���γ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���в��ϵĺϳɣ��������Ļ�ѧ��Ӧ�������������ֲ�ͬ����(����)
A������ϩ���� B��������ϩ����
C����ȩ��֬ D���۱���ϩ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D����ѧ�����Ļ���������ᴿ�Ļ���װ�á�
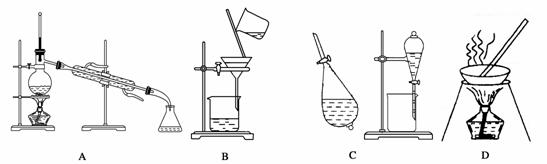
����ݻ���������ᴿ��ԭ�����ش�������ʵ������Ҫʹ������װ�á���A��B��C��D�����ʵ��Ŀո��С�
(1)��ȥCa(OH)2��Һ��������CaCO3__________________________________________��
(2)�ӵ�ˮ����ȡ��__________________________________________��
(3)������ˮ��ȡ����ˮ___________________________________��
(4)����ֲ���ͺ�ˮ_______________________________________��
(5)��ȥ�����е���ɳ__________________________________________��
(6)�뺣ˮɹ��ԭ���������__________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com