
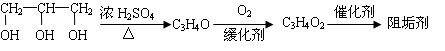
 ____��
____��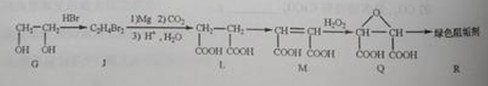
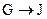 Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��
Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��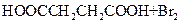
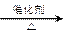
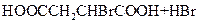 ��_____��_____���û�ѧ����ʽ��ʾ����
��_____��_____���û�ѧ����ʽ��ʾ����
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
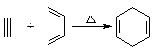 �����Ҫ�ϳ�
�����Ҫ�ϳ� �����õ�ԭ�Ͽ����� ��
�����õ�ԭ�Ͽ����� �� 
 F���������������У���ṹ��ʽ
F���������������У���ṹ��ʽ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
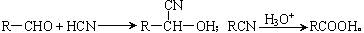
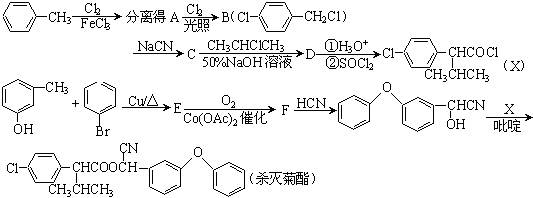

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
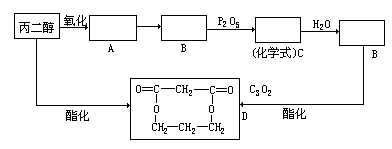
 ���ס��ҡ������DZ��ͻ������ҷ����ж�����26�����ӡ��ݴ��ƶϣ�
���ס��ҡ������DZ��ͻ������ҷ����ж�����26�����ӡ��ݴ��ƶϣ� ��Fԭ�Ӵ��棬���ò��������_______�ֽṹ��
��Fԭ�Ӵ��棬���ò��������_______�ֽṹ�� �� B������ӹ���Ϊ���ı���
�� B������ӹ���Ϊ���ı����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
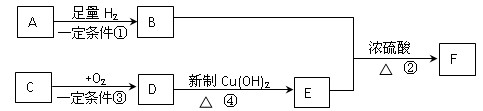
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
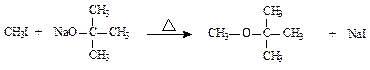
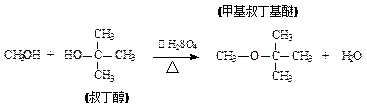 |
 ( )
( )| A��2��ʵ�鷽���У��������ƵõIJ���������������ڷ��� |
| B�������еõ���2�ֲ������ˮϴ��Һ������ |
| C�����嶡����һ�������2�� |
| D�������嶡���Ѻ��е��嶡�������ý����Ƴ�ȥ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com