
![]()
![]()
ͼ2-3-4
(1)д����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ________________________________________��
(2)��֪����������ʳ�ε��ܽ��Ϊ36.5 g������¶��±���ʳ��ˮ���ʵ���������Ϊ__________��
(3)��ÿ̨����ƽ��ÿ������2.3��104 molʳ�Σ�������ɵ������������������1��1.15ͨ��ϳ�¯�������Ǹ��ε�������ģ������Ͽ�����36.5%������__________t��
(4)������������1��a(a��1)ͨ��ϳ�¯����ó�ÿ������ʳ��c t�������Ǹ��ε�������ģ���ÿ������36.5%������__________b t��b=__________t��
������(2)���ڸ��¶���NaCl���ܽ��Ϊ36.5 g�����Ը��¶��±���NaCl����������Ϊ![]() ��100%=26.8%��
��100%=26.8%��
(3)�ɻ�ѧ����ʽ2NaCl+2H2O![]() 2NaOH+Cl2��+H2����H2+Cl2
2NaOH+Cl2��+H2����H2+Cl2![]() 2HCl��֪����ʹCl2��H2�������Ϊ1��1.15ͨ��ϳ����������1.15 mol H2����2.3 mol NaCl�ı�����Һ����ȫ��Ӧ�����2 mol HCl��������36.5%�����������Ϊx����
2HCl��֪����ʹCl2��H2�������Ϊ1��1.15ͨ��ϳ����������1.15 mol H2����2.3 mol NaCl�ı�����Һ����ȫ��Ӧ�����2 mol HCl��������36.5%�����������Ϊx����
2.3 NaCl �� 2HCl
2.3 mol 2��36.5 g
2.3��104 mol x��36.5%
x=![]()
(4) ��Cl2��������1��a(a��1)ͨ��ϳ�¯����(3)�еķ����ɵ����¹�ϵ��
aNaCl �� HCl
58.5a 36.5
c��106 g b��36.5%��106
��֮����![]() ��
��
�𰸣�(1)2NaCl+2H2O![]() 2NaOH+Cl2��+H2��
2NaOH+Cl2��+H2��
(2)26.8% (3)2.0 (4)![]()



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����������ƺ��ѧ�߶�12���¿���ѧ�Ծ����������� ���ͣ������
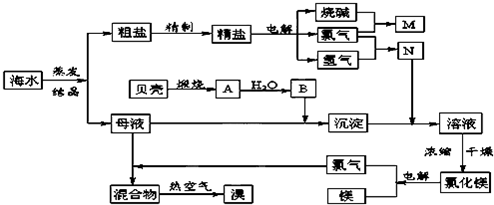
��10�֣�������ij�������Ӻ�ˮ����ȡNaCl��Mg����MgCl2��ʽ���ڣ���Br2����NaBr����ʽ���ڣ����ۺ����õ��������̼�ͼ��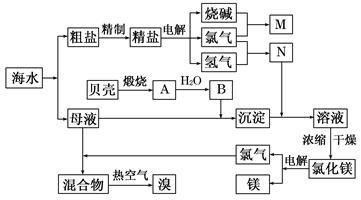
��1���ڴ����к���Ca2+��Mg2+��SO42�������ʣ�����ʱ���õ��Լ�Ϊ��
A������ B���Ȼ�����Һ C������������Һ ��D��̼������Һ��������Լ���˳���ǣ����ţ� �� ��
��2��Ŀǰ��ҵ����Ҫ�������ӽ���Ĥ����ⱥ��ʳ��ˮ������������� ������
A�����Ʊ���ʳ��ˮ����������
B����ˮ��������NaOH������������
C����������Ϊ�������ƺ�����
D�����۵������ý��������Ƴ�
��3��д����ⱥ��NaCl��Һ��������Ӧ����ʽ�� �� ��
��4������MgCl2�� 6H2O���Ƶ���ˮ�Ȼ�þ��Ӧ��ȡ�Ĵ�ʩ�� ��
��5�����ȿ�������������������������������Һ���գ�д����Ӧ�Ļ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������и߶�12���¿���ѧ�Ծ��������棩 ���ͣ������
��10�֣�������ij�������Ӻ�ˮ����ȡNaCl��Mg����MgCl2��ʽ���ڣ���Br2����NaBr����ʽ���ڣ����ۺ����õ��������̼�ͼ��
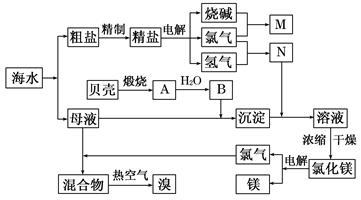
��1���ڴ����к���Ca2+��Mg2+��SO42�������ʣ�����ʱ���õ��Լ�Ϊ��
A������ B���Ȼ�����Һ C������������Һ ��D��̼������Һ��������Լ���˳���ǣ����ţ� �� ��
��2��Ŀǰ��ҵ����Ҫ�������ӽ���Ĥ����ⱥ��ʳ��ˮ������������� ������
A�����Ʊ���ʳ��ˮ����������
B����ˮ��������NaOH������������
C����������Ϊ�������ƺ�����
D�����۵������ý��������Ƴ�
��3��д����ⱥ��NaCl��Һ��������Ӧ����ʽ�� �� ��
��4������MgCl2�� 6H2O���Ƶ���ˮ�Ȼ�þ��Ӧ��ȡ�Ĵ�ʩ�� ��
��5�����ȿ�������������������������������Һ���գ�д����Ӧ�Ļ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com