
��10�֣���������������ֹʹ�������á��״��������ڦ�-Al2O3�������������ɼ����ѣ�CH3��S��CH3��������������NO2��Ӧ��ȡ�������� ( )���йط�Ӧ���£�
)���йط�Ӧ���£�
��Ӧ�� 2CH3OH(l)��H2S(g)��(CH3)2S(l) ��2H2O(l) ��H���DakJ��mol-1
��Ӧ�� (CH3)2S(l)��NO2(g)��(CH3)2SO(l)��NO(g) ��H����bkJ����mol-1
��Ӧ�� 2NO(g)��O2(g)��2NO2(g) ��H����ckJ��mol-1
��1��д���ü�����ֱ�Ӻ�������Ӧ��ȡ�����������Ȼ�ѧ��Ӧ����ʽ
___________________________________________________��
 ��2����˵����Ӧ2CH3OH(l)��H2S(g)
(CH3)2S(l) ��2H2O(l)��ƽ��״̬����____________
��
��2����˵����Ӧ2CH3OH(l)��H2S(g)
(CH3)2S(l) ��2H2O(l)��ƽ��״̬����____________
��
A. v(CH3OH) = 2v(H2S)
B. ���������У���ϵ��ѹǿ���ٸı�
C. ���������У���ϵ��������ܶȲ��ٸı�
D. ���������У������Ħ���������ٸı�
��3����Ӧ����һ�������¿ɴﵽƽ�⣬��������¸÷�Ӧƽ�ⳣ������ʽK= ____________________��
��4��N2O5��һ��������ɫ�����������Ʊ��������������֡�
����һ��4NO2(g)��O2(g) ��2N2O5(g)�� ��H����56.76 KJ��mol-1 [��Դ:ѧ#��#��Z#X#X#K]
�����£��÷�Ӧ�������Է����У�������Ӧ�ġ�S __________ 0�����������������
�������������⻯��ȼ�ϵ������Դ�����õ�ⷨ�Ʊ��õ�N2O5������ԭ������ͼ��

���⻯��ȼ�ϵ�ص�������Ӧʽ________________________��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 �����йط�Ӧ���£�
�����йط�Ӧ���£�| c2(NO2) |
| C2(NO)c(O2) |
| c2(NO2) |
| C2(NO)c(O2) |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������
��������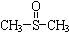 ������ֹʹ�������ã��״��������ڦ�-Al2O3�������������ɼ����ѣ�CH3-S-CH3��������������NO2��Ӧ��ȡ����������
������ֹʹ�������ã��״��������ڦ�-Al2O3�������������ɼ����ѣ�CH3-S-CH3��������������NO2��Ӧ��ȡ���������� �����йط�Ӧ���£�
�����йط�Ӧ���£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���������������ֹʹ�������á��״��������ڦ�-Al2O3�������������ɼ����ѣ�CH3��S��CH3��������������NO2��Ӧ��ȡ�������� ( )���йط�Ӧ���£�
��Ӧ�� 2CH3OH(l)��H2S(g)��(CH3)2S(l) ��2H2O(l) ��H���DakJ��mol-1
��Ӧ�� (CH3)2S(l)��NO2(g)��(CH3)2SO(l)��NO(g) ��H����bkJ����mol-1
��Ӧ�� 2NO(g)��O2(g)��2NO2(g) ��H����ckJ��mol-1
��1��д���ü�����ֱ�Ӻ�������Ӧ��ȡ�����������Ȼ�ѧ��Ӧ����ʽ
___________________________________________________��
![]() ��2����˵����Ӧ2CH3OH(l)��H2S(g) (CH3)2S(l) ��2H2O(l)��ƽ��״̬����____________��
��2����˵����Ӧ2CH3OH(l)��H2S(g) (CH3)2S(l) ��2H2O(l)��ƽ��״̬����____________��
A. v(CH3OH) =2v(H2S)
B. ���������У���ϵ��ѹǿ���ٸı�
C. ���������У���ϵ��������ܶȲ��ٸı�
D. ���������У������Ħ���������ٸı�
��3����Ӧ����һ�������¿ɴﵽƽ�⣬��������¸÷�Ӧƽ�ⳣ������ʽK= ____________________��
��4��N2O5��һ��������ɫ�����������Ʊ��������������֡�
����һ��4NO2(g)��O2(g) ��2N2O5(g)����H����56.76KJ��mol-1
�����£��÷�Ӧ�������Է����У�������Ӧ�ġ�S __________ 0�����������������
�������������⻯��ȼ�ϵ������Դ�����õ�ⷨ�Ʊ��õ�N2O5������ԭ������ͼ��
���⻯��ȼ�ϵ�ص�������Ӧʽ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�콭��ʡ�����������и������в��ԣ�һ����ѧ�Ծ� ���ͣ������
��10�֣���������������ֹʹ�������á��״��������ڦ�-Al2O3�������������ɼ����ѣ�CH3��S��CH3��������������NO2��Ӧ��ȡ�������� ( )���йط�Ӧ���£�
)���йط�Ӧ���£�
��Ӧ�� 2CH3OH(l)��H2S(g)��(CH3)2S(l) ��2H2O(l) ��H���DakJ��mol-1
��Ӧ�� (CH3)2S(l)��NO2(g)��(CH3)2SO(l)��NO(g) ��H����bkJ����mol-1
��Ӧ�� 2NO(g)��O2(g)��2NO2(g) ��H����ckJ��mol-1
��1��д���ü�����ֱ�Ӻ�������Ӧ��ȡ�����������Ȼ�ѧ��Ӧ����ʽ
___________________________________________________�� ��2����˵����Ӧ2CH3OH(l)��H2S(g) (CH3)2S(l) ��2H2O(l)��ƽ��״̬����____________ ��
��2����˵����Ӧ2CH3OH(l)��H2S(g) (CH3)2S(l) ��2H2O(l)��ƽ��״̬����____________ ��
| A��v(CH3OH) =" 2v(H2S)" |
| B�����������У���ϵ��ѹǿ���ٸı� |
| C�����������У���ϵ��������ܶȲ��ٸı� |
D�����������У������Ħ�������� �ٸı� �ٸı� |
 ����H����56.76 KJ��mol-1
����H����56.76 KJ��mol-1 
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com