
”¾ĢāÄæ”æ»Ų“šŅŌĻĀĪŹĢā£ŗ
£Ø1£©½«ŅŃČ„³ż±ķĆęŃõ»ÆĪļµÄĢś¶¤(Ģ¼ĖŲøÖ)·ÅČėĄäÅØĮņĖįÖŠ£¬10·ÖÖÓŗóŅĘČėĮņĖįĶČÜŅŗÖŠ£¬Ę¬æĢŗóČ”³ö¹Ū²ģ£¬Ģś¶¤±ķĆęĪŽĆ÷ĻŌ±ä»Æ£¬ĘäŌŅņŹĒ__________________________”£
£Ø2£©Įķ³ĘČ”ŅŃČ„³ż±ķĆęŃõ»ÆĪļµÄĢś¶¤(Ģ¼ĖŲøÖ)6£®0g·ÅČė15£®0mlÅØĮņĖįÖŠ£¬¼ÓČČ£¬³ä·ÖÓ¦ŗóµĆµ½ČÜŅŗX²¢ŹÕ¼Æµ½ĘųĢåY”£
¢Ł¼×Ķ¬Ń§ČĻĪŖXÖŠ³żFe3+Ķā»¹æÉÄÜŗ¬ÓŠFe2+”£Š“³öÉś³ÉFe2+ĖłÓŠæÉÄܵĥė×Ó·½³ĢŹ½£ŗ______________”£
¢ŚŅŅĶ¬Ń§Č”336mL(±ź×¼×“æö)ĘųĢåYĶØČė×ćĮæĀČĖ®ÖŠ£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ______________”£
Č»ŗó¼ÓČė×ćĮæBaCl2ČÜŅŗ£¬¾ŹŹµ±²Ł×÷ŗóµĆøÉŌļ¹ĢĢå2£®33g”£ÓÉÓŚ“ĖĶĘÖŖĘųĢåYÖŠSO2µÄĢå»ż·ÖŹżĪŖ___________”£
£Ø3£©Ļņ100mLĖ®ÖŠĶ¶ČėNaŗĶAl¹²16æĖ£¬³ä·Ö·“Ó¦ŗóŹ£Óą½šŹō1g”£¼ĘĖć·Å³öH2µÄĢå»żĪŖ________Éż(±ź×¼×“æöĻĀ)
£Ø4£©Ļņŗ¬ÓŠ0.3mol NaAlO2µÄČÜŅŗÖŠµĪ¼Ó1mol/L HCl£¬µ±Éś³É7.8æĖ³ĮµķŹ±£¬¼ÓČėŃĪĖįµÄĢå»żĪŖ________mL
£Ø5£©ĻņŅ»¶ØĮæµÄFe”¢Fe2O3ŗĶCuO»ģŗĻĪļĶ¶Čė120 ml 2.2 mol/LµÄĮņĖįČÜŅŗÖŠ£¬³ä·Ö·“Ó¦ŗóÉś³É896 mL±ź×¼×“æöĻĀµÄĘųĢ壬µĆ²»ČÜĪļ1.28 g£¬¹żĀĖŗó£¬ĻņĀĖŅŗÖŠ¼ÓČė2 mol/LµÄNaOHČÜŅŗ£¬¼ÓÖĮ40 mLŹ±æŖŹ¼³öĻÖ³Įµķ£¬ŌņĀĖŅŗÖŠFeSO4µÄĪļÖŹµÄĮæÅضČĪŖ(ÉčĀĖŅŗĢå»żĪŖ120 ml)_________mol/L
”¾“š°ø”æĢś±ķĆę±»¶Ū»Æ Fe+2H+£½Fe2++H2”ü£»Fe+ 2Fe3+£½3Fe2+ SO2+Cl2+2H2O£½2HCl+H2SO4 66£®7% 13.44 100»ņ900 1.87mol”¤L£1
”¾½āĪö”æ
£Ø1£©Ģś¶¤(Ģ¼ĖŲøÖ)·ÅČėĄäÅØĮņĖįÖŠ£¬ÅØĮņĖįÓŠ½ĻĒæµÄŃõ»ÆŠŌÄÜŹ¹Ģś¶¤¶Ū»Æ×čÖ¹·“Ó¦½ųŅ»²½½ųŠŠ£¬¹Ź“š°øĪŖĢś±ķĆę±»¶Ū»Æ£»
£Ø2£©¢ŁŌŚ¼ÓČČĢõ¼žĻĀ£¬ÅØĮņĖįÓėFe·“Ӧɜ³ÉFe3+£¬µ±ČÜŅŗÅØ¶Č¼õŠ”Ź±£¬ĮņĖįŃõ»ÆŠŌ¼õČõ£¬Éś³ÉFe2+£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖFe+2H+=Fe2++H2”ü£¬ČēĮņĖįĶźČ«ĻūŗÄ£¬ĒŅĢś¹żĮæŹ±£¬»¹»į·¢ÉśFe+2Fe3+=3Fe2+£¬¹Ź“š°øĪŖFe+2H+=Fe2++H2”ü£» Fe+2Fe3+=3Fe2+£»
¢ŚSO2¾ßÓŠ»¹ŌŠŌ£¬ĶØČė×ćĮæäåĖ®ÖŠ£¬·¢ÉśSO2+Br2+2H2O=2HBr+H2SO4£¬n(»ģŗĻĘųĢå)=![]() =0.015mol£¬øł¾ŻĮņŹŲŗć£ŗn(SO2)=n(BaSO4)=
=0.015mol£¬øł¾ŻĮņŹŲŗć£ŗn(SO2)=n(BaSO4)=![]() =0.01mol£¬ŌņSO2µÄĢå»ż·ÖŹż£ŗ
=0.01mol£¬ŌņSO2µÄĢå»ż·ÖŹż£ŗ![]() ”Į100%=66.7%£¬¹Ź“š°øĪŖSO2+Br2+2H2O=2HBr+H2SO4£»66.7%”£
”Į100%=66.7%£¬¹Ź“š°øĪŖSO2+Br2+2H2O=2HBr+H2SO4£»66.7%”£
£Ø3£©AlŗĶĖ®²»·“Ó¦£¬ĖłŅŌŹ£Óą½šŹōÓ¦øĆŹĒAl£¬·“Ó¦·½³ĢŹ½ĪŖ2Na+2H2O=2NaOH+H2”ü£¬2Al+2NaOH+2H2O=2NaAlO2+3H2”ü£¬Į½·“Ó¦Ļą¼Ó£ŗAl+Na+2H2O=2NaAlO2+2H2”ü£¬Ōņ²ĪÓė·“Ó¦µÄNaŗĶAlµÄÖŹĮæÖ®±ČĪŖ23£ŗ27£¬NaŗĶAlµÄÖŹĮæŗĶĪŖ15g£¬ĖłŅŌn(Na)=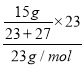 =0.3mol£¬n(Al)=0.3mol£¬n(H2)=2n(Na)=0.03mol£¬V(H2)=0.03mol”Į22.4L/mol=13.44L£¬¹Ź“š°øĪŖ13.44£»
=0.3mol£¬n(Al)=0.3mol£¬n(H2)=2n(Na)=0.03mol£¬V(H2)=0.03mol”Į22.4L/mol=13.44L£¬¹Ź“š°øĪŖ13.44£»
£Ø4£©n(Al(OH)3)=![]() =0.1mol£¼0.3mol£¬Ņ»ÖÖĒéæöŹĒ£ŗĀĮŌŖĖŲ“ęŌŚÓŚĘ«ĀĮĖįÄĘŗĶĒāŃõ»ÆĀĮÖŠ£¬“ĖŹ±ŃĪĖįÉŁĮ棬ֻÄܳĮµķŅ»²æ·ÖAlO2-£¬øł¾ŻNaAlO2+HCl+H2O=Al(OH)3”ż+NaClµĆ£¬n(HCl)=n(Al(OH)3)=0.1mol£¬ŌņŃĪĖįĢå»ż=
=0.1mol£¼0.3mol£¬Ņ»ÖÖĒéæöŹĒ£ŗĀĮŌŖĖŲ“ęŌŚÓŚĘ«ĀĮĖįÄĘŗĶĒāŃõ»ÆĀĮÖŠ£¬“ĖŹ±ŃĪĖįÉŁĮ棬ֻÄܳĮµķŅ»²æ·ÖAlO2-£¬øł¾ŻNaAlO2+HCl+H2O=Al(OH)3”ż+NaClµĆ£¬n(HCl)=n(Al(OH)3)=0.1mol£¬ŌņŃĪĖįĢå»ż=![]() =100mL£»
=100mL£»
ĮķŅ»ÖÖĒéæöŹĒĀĮŌŖĖŲ“ęŌŚÓŚĀČ»ÆĀĮŗĶĒāŃõ»ÆĀĮÖŠ£¬“ĖŹ±ŃĪĖį½Ļ¶ą£¬ĻČ°ŃAlO2-Č«²æ³Įµķ£¬ŗóÓÖ½«²æ·Ö³ĮµķČܽā£¬Éś³ÉĒāŃõ»ÆĀĮŠčŅŖn(HCl)=n(NaAlO2)=n(Al(OH)3)=0.3mol£¬ČܽāAl(OH)3Éś³ÉĀČ»ÆĀĮŠčŅŖŃĪĖįµÄn(HCl)=3n(AlCl3)=3”Į(0.3-0.1)mol=0.6mol£¬ĖłŅŌŠčŅŖŃĪĖįĢå»ż=![]() =900mL£¬¹Ź“š°øĪŖ100»ņ900£»
=900mL£¬¹Ź“š°øĪŖ100»ņ900£»
£Ø5£©¹ĢĢå»ģŗĻĪļŗĶĻ”ĮņĖį·“Ó¦ŗóÓŠ¹ĢĢåŹ£Óą£¬ĖµĆ÷ČÜŅŗÖŠ²»“ęŌŚĢśĄė×Ó£¬ĻņĀĖŅŗÖŠ¼ÓČė¼ÓČė2mol/LµÄNaOHČÜŅŗ£¬¼ÓÖĮ40mLŹ±æŖŹ¼³öĻÖ³Įµķ£¬ĖµĆ÷ĮņĖįÓŠŹ£Óą£¬ĢśĻČŗĶĶĄė×ÓŗóŗĶĒāĄė×Ó·¢ÉśÖĆ»»·“Ó¦£¬ĖłŅŌĀĖŅŗÖŠµÄČÜÖŹŹĒĮņĖį”¢ĮņĖįŃĒĢś£¬n(H2SO4)=2.2mol/L”Į0.12L=0.264mol£¬
ÓėĒāŃõ»ÆÄĘ·“Ó¦µÄĮņĖįµÄĪļÖŹµÄĮæ=![]() n(NaOH)=
n(NaOH)=![]() ”Į2mol/L”Į0.04L=0.04mol£¬
”Į2mol/L”Į0.04L=0.04mol£¬
Ź£ÓąµÄĮņĖįµÄĪļÖŹµÄĮæ=0.264mol-0.04mol=0.224mol£¬
Ź£ÓąĮņĖįÓė¹ĢĢå·“Ó¦Éś³ÉĮĖĮņĖįŃĒĢś£¬øł¾ŻĮņĖįŗĶĮņĖįŃĒĢśµÄ¹ŲĻµŹ½¼ĘĖćĮņĖįŃĒĢśµÄĪļÖŹµÄĮæÅضČ=![]() =1.87mol/L£¬¹Ź“š°øĪŖ1.87”£
=1.87mol/L£¬¹Ź“š°øĪŖ1.87”£


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ![]() µÄ
µÄ![]() ČÜŅŗ¼ÓĖ®Ļ”ŹĶ100±¶ŗó£¬pH______
ČÜŅŗ¼ÓĖ®Ļ”ŹĶ100±¶ŗó£¬pH______![]() Ģī”°
Ģī”°![]() ”±”°
”±”°![]() ”±»ņ”°
”±»ņ”°![]() ”±
”±![]() £¬ŌŅņŹĒ______
£¬ŌŅņŹĒ______![]() ÓĆĄė×Ó·½³ĢŹ½ŗĶ±ŲŅŖµÄĪÄ×ÖĖµĆ÷
ÓĆĄė×Ó·½³ĢŹ½ŗĶ±ŲŅŖµÄĪÄ×ÖĖµĆ÷![]() £»pHĻąµČµÄNaOHČÜŅŗÓė
£»pHĻąµČµÄNaOHČÜŅŗÓė![]() ČÜŅŗ£¬·Ö±š¼ÓČȵ½ĻąĶ¬µÄĪĀ¶Čŗó
ČÜŅŗ£¬·Ö±š¼ÓČȵ½ĻąĶ¬µÄĪĀ¶Čŗó![]() ČÜŅŗµÄpH______NaOHČÜŅŗµÄ
ČÜŅŗµÄpH______NaOHČÜŅŗµÄ![]() Ģī”°
Ģī”°![]() ”±”°
”±”°![]() ”±»ņ”°
”±»ņ”°![]() ”±
”±![]() £»
£»
![]() ĻąµČŹ±£¬
ĻąµČŹ±£¬![]() ČżÖÖČÜŅŗÖŠ
ČżÖÖČÜŅŗÖŠ![]() Óɓ󵽊”µÄĖ³ŠņĪŖ______£»
Óɓ󵽊”µÄĖ³ŠņĪŖ______£»
![]() µČĢå»ż”¢µČÅØ¶ČµÄĒāŃõ»ÆÄĘÓė“×Ėį»ģŗĻŗóČÜŅŗ³Ź ______ ŠŌ£¬ČÜŅŗÖŠ
µČĢå»ż”¢µČÅØ¶ČµÄĒāŃõ»ÆÄĘÓė“×Ėį»ģŗĻŗóČÜŅŗ³Ź ______ ŠŌ£¬ČÜŅŗÖŠ![]() ______
______ ![]() Ģī”°
Ģī”°![]() ”±”°
”±”°![]() ”±»ņ”°
”±»ņ”°![]() ”±
”±![]() £»
£»![]() µÄĒāŃõ»ÆÄĘÓė
µÄĒāŃõ»ÆÄĘÓė![]() µÄ“×ĖįµČĢå»ż»ģŗĻŗóČÜŅŗ³Ź______ŠŌ£¬ČÜŅŗÖŠ
µÄ“×ĖįµČĢå»ż»ģŗĻŗóČÜŅŗ³Ź______ŠŌ£¬ČÜŅŗÖŠ![]() ______
______ ![]() Ģī”°
Ģī”°![]() ”±”°
”±”°![]() ”±»ņ”°
”±»ņ”°![]() ”±
”±![]() £»
£»
![]() £¬ÓĆ
£¬ÓĆ![]() ČÜŅŗµĪ¶Ø
ČÜŅŗµĪ¶Ø![]() ijŅ»ŌŖĖįHAČÜŅŗĖłµĆµĪ¶ØĒśĻßČēĶ¼”£
ijŅ»ŌŖĖįHAČÜŅŗĖłµĆµĪ¶ØĒśĻßČēĶ¼”£
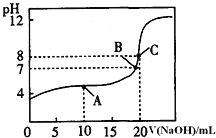
![]() ĪŖ¼õŠ”ŹµŃéĪó²ī£¬ÓÉĶ¼æÉÖŖµĪ¶ØŹ±ÖøŹ¾¼ĮӦєÓĆ______
ĪŖ¼õŠ”ŹµŃéĪó²ī£¬ÓÉĶ¼æÉÖŖµĪ¶ØŹ±ÖøŹ¾¼ĮӦєÓĆ______![]() Ģī”°ŹÆČļ”±”¢”°·ÓĢŖ”±”¢”°¼×»ł³Č”±
Ģī”°ŹÆČļ”±”¢”°·ÓĢŖ”±”¢”°¼×»ł³Č”±![]() £»
£»
![]() ”¢B”¢CČżµćĖłŹ¾ČÜŅŗµ¼µēÄÜĮ¦×īĒæµÄŹĒ ______ µć¶ŌÓ¦µÄČÜŅŗ£»
”¢B”¢CČżµćĖłŹ¾ČÜŅŗµ¼µēÄÜĮ¦×īĒæµÄŹĒ ______ µć¶ŌÓ¦µÄČÜŅŗ£»
![]() £¬AµćµÄĄė×ÓÅØ¶Č“óŠ”¹ŲĻµŹĒ ______ £®
£¬AµćµÄĄė×ÓÅØ¶Č“óŠ”¹ŲĻµŹĒ ______ £®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓŠ»śĪļMµÄ½į¹¹¼ņŹ½ĪŖ£ŗÓŠ¹ŲMµÄĻĀĮŠŠšŹöÖŠÕżČ·µÄŹĒ£Ø £©
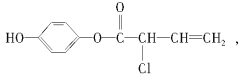
A£®æÉÓėH2·“Ó¦£¬1 mol M×ī¶ąĻūŗÄ5 mol H2
B£®æÉÓėÅØäåĖ®·“Ó¦£¬1 mol M×ī¶ąĻūŗÄ4 mol Br2
C£®æÉÓėNaOHČÜŅŗ·“Ó¦£¬1 mol M×ī¶ąĻūŗÄ4 mol NaOH
D£®M²»ÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¹żŃõ»ÆÄĘ³£×÷ĘÆ°×¼Į”¢É±¾ś¼Į”¢Ļū¶¾¼Į”£¹żŃõ»ÆÄʱ£“ę²»µ±ČŻŅ×ĪüŹÕæÕĘųÖŠCO£²¶ų±äÖŹ”£
£Ø1£©Ä³æĪĶā»ī¶ÆŠ”×éÓūĢ½¾æij¹żŃõ»ÆÄĘѳʷŹĒ·ńŅŃ¾±äÖŹ£¬Č”ÉŁĮæѳʷ£¬Čܽā£¬¼ÓČė__________ČÜŅŗ£¬³ä·ÖÕńµ“ŗóÓŠ°×É«³Įµķ£¬Ö¤Ć÷Na2O2ŅŃ¾±äÖŹ”£
£Ø2£©øĆæĪĶā»ī¶ÆŠ”×éĪŖĮĖ“ÖĀŌ²ā¶Ø¹żŃõ»ÆÄʵēæ¶Č£¬ĖūĆĒ³ĘČ”a gѳʷ£¬²¢Éč¼ĘÓĆĻĀĶ¼×°ÖĆĄ“²ā¶Ø¹żŃõ»ÆÄʵÄÖŹĮæ·ÖŹż”£
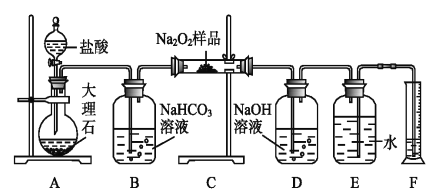
¢ŁAÖŠ·¢Éś·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖ_____________________”£
¢Ś½«ŅĒĘ÷Į¬½ÓŗĆŅŌŗ󣬱ŲŠė½ųŠŠµÄµŚŅ»²½²Ł×÷ŹĒ_____________________”£
¢ŪB×°ÖĆ³öĄ“µÄĘųĢåŹĒ·ńŠčŅŖøÉŌļ_________________”££ØĢī”°ŹĒ”±»ņ”°·ń”±£©
¢ÜŠ“³ö×°ÖĆCÖŠ·¢ÉśµÄĖłÓŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½_____________£¬______________”£
¢ŻDÖŠNaOHČÜŅŗµÄ×÷ÓĆ_______________________”£
¢ŽŹµŃé½įŹųŹ±£¬¶ĮČ”ŹµŃéÖŠÉś³ÉĘųĢåµÄĢå»żŹ±£¬²»ŗĻĄķµÄŹĒ_______________”£
a.Ö±½Ó¶ĮČ”ĘųĢåĢå»ż£¬²»ŠčĄäČ“µ½ŹŅĪĀ
b.ÉĻĻĀŅʶÆĮæĶ²£¬Ź¹µĆE”¢FÖŠŅŗĆęø߶ČĻąĶ¬
c.ŹÓĻßÓė°¼ŅŗĆęµÄ×īµĶµćĻąĘ½¶ĮČ”ĮæĶ²ÖŠĖ®µÄĢå»ż
¢ß¶Į³öĮæĶ²ÄŚĖ®µÄĢå»żŗó£¬ÕŪĖć³É±ź×¼×“æöĻĀŃõĘųµÄĢå»żĪŖV mL£¬Ōņѳʷ֊¹żŃõ»ÆÄʵÄÖŹĮæ·ÖŹżĪŖ__________________”£
¢ąŹµŃéĶź³ÉŗóEµ½FÖ®¼äµ¼¹ÜÄŚ²ŠĮōĖ®µÄĢå»ż»įŹ¹²āĮæ½į¹ū__________”££ØĢī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»Ó°Ļģ”±£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¢ń£®ŹµŃéŹŅÖʵĆĘųĢåÖŠ³£ŗ¬ÓŠŌÓÖŹ£¬Ó°ĻģĘäŠŌÖŹµÄ¼ģŃ锣ĻĀĶ¼AĪŖ³żŌÓ×°ÖĆ£¬BĪŖŠŌÖŹ¼ģŃé×°ÖĆ£¬Ķź³ÉĻĀĮŠ±ķøń£ŗ
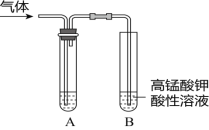
ŠņŗÅ | ĘųĢå | ·“Ó¦ŌĄķ | AÖŠŹŌ¼Į |
¢Ł | ŅŅĻ© | ĪŽĖ®ŅŅ“¼ÓėÅØĮņĖį¹²ČČ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ______ | ___ |
¢Ś | ŅŅĻ© | äåŅŅĶéÓėNaOHµÄŅŅ“¼ČÜŅŗ¹²ČČ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_____ | ___ |
¢Ū | ŅŅČ² | ĻņµēŹÆÖŠµĪ¼Ó±„ŗĶŹ³ŃĪĖ®£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ___ | ____ |
¢ņ£®ĪŖĢ½¾æŅŅĖįŅŅõ„µÄĖ®½āĒéæö£¬Ä³Ķ¬Ń§Č”“óŠ”ĻąĶ¬µÄ3Ö§ŹŌ¹Ü£¬·Ö±š¼ÓČėŅŌĻĀČÜŅŗ£¬³ä·ÖÕńµ“£¬·ÅŌŚĶ¬Ņ»Ė®Ō”ÖŠ¼ÓČČĻąĶ¬Ź±¼ä£¬¹Ū²ģµ½ČēĻĀĻÖĻó”£
ŹŌ¹Ü±ąŗÅ | ¢Ł | ¢Ś | ¢Ū |
ŹµŃé²Ł×÷ |
|
|
|
ŹµŃéĻÖĻó | õ„²ć±ä±” | õ„²ćĻūŹ§ | õ„²ć»ł±¾²»±ä |
£Ø1£©ŹŌ¹Ü¢ŚÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ__________”£
£Ø2£©Éč¼ĘŹŌ¹Ü¢ŪŹµŃéµÄ×÷ÓĆŹĒ__________”£
£Ø3£©ŹµŃé½įĀŪŹĒ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĪļÖŹXµÄ½į¹¹¼ņŹ½ČēĶ¼ĖłŹ¾£¬Ėü³£ÓĆÓŚÖĘĻćĮĻ»ņ×÷ĪŖŅūĮĻĖį»Æ¼Į£¬ŌŚŅ½Ń§ÉĻŅ²ÓŠ¹ć·ŗÓĆĶ¾”£ĻĀĮŠ¹ŲÓŚĪļÖŹXµÄĖµ·ØÕżČ·µÄŹĒ£Ø £©
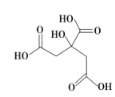
A.XµÄ·Ö×ÓŹ½ĪŖC6H7O7
B.X·Ö×ÓÄŚĖłÓŠŌ×Ó¾łŌŚĶ¬Ņ»Ę½ĆęÄŚ
C.1molĪļÖŹX×ī¶ąæÉŅŌŗĶ3molĒāĘų·¢Éś¼Ó³É·“Ó¦
D.×ćĮæµÄX·Ö±šÓėµČĪļÖŹµÄĮæµÄNaHCO3”¢Na2CO3·“Ó¦µĆµ½µÄĘųĢåµÄĪļÖŹµÄĮæĻąĶ¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹµŃéŹŅÓĆ»ÆŗĻĪļAÄ£Äā¹¤ŅµÉĻÖʱøŗ¬ŃõĖįDµÄ¹ż³ĢČēĶ¼ĖłŹ¾£¬ŅŃÖŖDĪŖĒæĖį£¬Ēė»Ų“šĻĀĮŠĪŹĢā”£
![]()
£Ø1£©ČōAŌŚ³£ĪĀĻĀĪŖ¹ĢĢ壬BŹĒÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĪŽÉ«ĘųĢ唣
¢ŁDµÄ»ÆѧŹ½ŹĒ________£»
¢ŚŌŚ¹¤ŅµÉś²śÖŠ£¬BĘųĢåµÄ“óĮæÅŷű»ÓźĖ®ĪüŹÕŗóŠĪ³ÉĮĖ_____¶ųĪŪČ¾ĮĖ»·¾³”£
£Ø2£©ČōAŌŚ³£ĪĀĻĀĪŖĘųĢ壬CŹĒŗģ×ŲÉ«µÄĘųĢ唣
¢ŁAµÄ»ÆѧŹ½ŹĒ_________£»CµÄ»ÆѧŹ½ŹĒ_______”£
¢ŚDµÄÅØČÜŅŗŌŚ³£ĪĀĻĀæÉÓėĶ·“Ó¦²¢Éś³ÉCĘųĢ壬·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ____£¬øĆ·“Ó¦______£ØĢī”°ŹōÓŚ”±»ņ”°²»ŹōÓŚ”±£©Ńõ»Æ»¹Ō·“Ó¦”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅŅĖįŅŅõ„ŹĒÖŲŅŖµÄÓŠ»śŗĻ³ÉÖŠ¼äĢ壬¹ć·ŗÓ¦ÓĆÓŚ»Æѧ¹¤Ņµ”£ŹµŃéŹŅÖĘČ”ŅŅĖįŅŅõ„µÄÖ÷ŅŖ²½ÖčČēĻĀ£ŗ
¢ŁŌŚ¼×ŹŌ¹Ü(ČēĶ¼)ÖŠ¼ÓČė 2mL ÅØĮņĖį”¢3mL ŅŅ“¼ŗĶ 2mL ŅŅĖįµÄ»ģŗĻČÜŅŗ”£
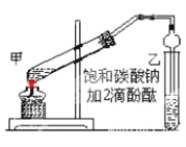
¢Ś°“ČēĶ¼Į¬½ÓŗĆ×°ÖĆ(×°ÖĆĘųĆÜŠŌĮ¼ŗĆ)²¢¼ÓČė»ģŗĻŅŗ£¬Š”»š¾łŌȵŲ¼ÓČČ 3-5min”£
¢Ū“żŹŌ¹ÜŅŅŹÕ¼Æµ½Ņ»¶ØĮæ²śĪļŗóĶ£Ö¹¼ÓČČ£¬³·³öŹŌ¹Ü²¢ÓĆĮ¦Õńµ“£¬Č»ŗó¾²ÖĆ“ż·Ö²ć”£
¢Ü·ÖĄė³öŅŅĖįŅŅõ„²ć”¢Ļ“µÓ”¢øÉŌļ”£
(1)ČōŹµŃéÖŠÓĆŅŅĖįŗĶŗ¬18O µÄŅŅ“¼×÷ÓĆ£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_____”£
(2)¼×ŹŌ¹ÜÖŠ£¬»ģŗĻČÜŅŗµÄ¼ÓČėĖ³Šņ£ŗ_____£»
(3)²½Öč¢ŚÖŠŠčŅŖÓĆŠ”»š¾łŌČ¼ÓČČ£¬ĘäÖ÷ŅŖŌŅņŹĒ___________£»
(4)ÉĻŹöŹµŃéÖŠ±„ŗĶĢ¼ĖįÄĘČÜŅŗµÄ×÷ÓĆŹĒ_____(Ģī×ÖÄø“śŗÅ)”£
A.·“Ó¦µōŅŅĖįŗĶŅŅ“¼
B.·“Ó¦µōŅŅĖį²¢ĪüŹÕŅŅ“¼
C.ŅŅĖįŅŅõ„ŌŚ±„ŗĶĢ¼ĖįÄĘČÜŅŗÖŠµÄČܽā¶Č±ČŌŚĖ®ÖŠøüŠ”£¬ÓŠĄūÓŚ·Ö²ćĪö³ö
D.¼ÓĖŁõ„µÄÉś³É£¬ĢįøßĘä²śĀŹ
(5)Óū½«ŅŅŹŌ¹ÜÖŠµÄĪļÖŹ·ÖĄėæŖŅŌµĆµ½ŅŅĖįŅŅõ„£¬±ŲŠėŹ¹ÓƵÄŅĒĘ÷ŹĒ_____£»·ÖĄėŹ±£¬ŅŅĖįŅŅõ„Ó¦øĆ“ÓŅĒĘ÷_____(Ģī£ŗ”°ĻĀæŚ·Å”±»ņ”°ÉĻæŚµ¹”±)³ö”£
(6)Éś³ÉŅŅĖįŅŅõ„µÄ·“Ó¦ŹĒæÉÄę·“Ó¦£¬·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬ĻĀĮŠĆčŹöÄÜĖµĆ÷ŅŅ“¼ÓėŅŅĖįµÄõ„»Æ·“Ó¦ŅŃ“ļµ½»ÆŃ§Ę½ŗāדĢ¬µÄÓŠ_____(ĢīŠņŗÅ)”£
¢Ł»ģŗĻĪļÖŠø÷ĪļÖŹµÄÅØ¶Č²»ŌŁ±ä»Æ£»
¢Śµ„Ī»Ź±¼äĄļ£¬Éś³É 1mol ŅŅ“¼£¬Ķ¬Ź±Éś³É 1mol ŅŅĖį£»
¢Ūµ„Ī»Ź±¼äĄļ£¬Éś³É 1mol ŅŅĖįŅŅõ„£¬Ķ¬Ź±Éś³É 1mol ŅŅĖį”£
(7)ČōĻÖÓŠŅŅĖį 90g£¬ŅŅ“¼ 138g ·¢Éśõ„»Æ·“Ó¦µĆµ½ 88g ŅŅĖįŅŅõ„£¬ŹŌ¼ĘĖćøĆ·“Ó¦µÄ²śĘ·²śĀŹĪŖ_____”£(²śĀŹ%=(Źµ¼Ź²śĮæ/ĄķĀŪ²śĮæ)”Į100%)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĮ×»ÆÅšŹĒŅ»ÖÖ³¬Ó²ÄĶÄ„Ķæ²ć²ÄĮĻ”£ĻĀĶ¼ĪŖĘ侧Ģå½į¹¹ÖŠ×īŠ”µÄÖŲø“½į¹¹µ„ŌŖ£¬ĘäÖŠµÄĆæøöŌ×Ó¾łĀś×ć8µē×ÓĪČ¶Ø½į¹¹”£ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ( )
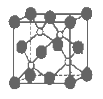
A.Į×»ÆÅš¾§ĢåµÄ»ÆѧŹ½ĪŖBP£¬ŹōÓŚĄė×Ó¾§Ģå
B.Į×»ÆÅš¾§ĢåµÄČŪµćøߣ¬ĒŅČŪȌדĢ¬ĻĀÄܵ¼µē
C.Į×»ÆÅš¾§ĢåÖŠĆæøöŌ×Ó¾łŠĪ³É4Ģõ¹²¼Ū¼ü
D.Į×»ÆÅš¾§Ģå½į¹¹Ī¢Į£µÄæÕ¼ä¶Ń»ż·½Ź½ÓėĀČ»ÆÄĘĻąĶ¬
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com