
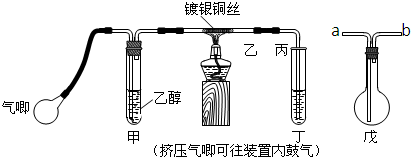
·ÖĪö £Ø1£©ŅŅ“¼Ņ×»Ó·¢£¬ÉżøßĪĀ¶ČÓŠĄūÓŚŅŅ“¼µÄ»Ó·¢£»
£Ø2£©¢ŁøĆ·“Ó¦Ņż·¢ŗ󣬲»Šč¼ÓČČ¼“æɽųŠŠ£¬ĖµĆ÷·“Ó¦ŹĒ·ÅČČµÄ£»
¢Śøł¾Ż¼×ÖŠµ„Ī»Ź±¼äÄŚµÄĘųÅŻŹżĄ“æŲÖĘĘųĮ÷£»
£Ø3£©ŅŅČ©Ņ×ČÜÓŚĖ®£¬ĪüŹÕŹ±Ó¦·ĄÖ¹µ¹ĪüµÄ·¢Éś£¬ŅŅČ©Ņ×»Ó·¢£¬²»ÓĆĖ®ĪüŹÕ¶ųŹĒÖ±½ÓĄäȓӦєŌń±łĖ®Ō”£»
£Ø4£©ŅŅČ©ŗĶŠĀÖĘCu£ØOH£©2µÄ·“Ó¦·¢ÉśŃõ»Æ·“Ó¦£¬ŅŅČ©·Ö×ÓÖŠµÄČ©»ł±»Ńõ»ÆĪŖōČ»ł£¬ĒāŃõ»ÆĶ±»»¹ŌĪŖŃõ»ÆŃĒĶŗģÉ«³Įµķ£®
½ā“š ½ā£ŗ£Ø1£©ŅŅ“¼¾ßÓŠ»Ó·¢ŠŌ£¬ÉżøßĪĀ¶ČÄÜ“Ł½ųŅŅ“¼µÄ»Ó·¢£¬Ź¹Éś³ÉŅŅ“¼ÕōĘųµÄĖŁĀŹ¼Óæģ£¬
¹Ź“š°øĪŖ£ŗ¼ÓČČ£¬¼Óæģ²śÉśŅŅ“¼ÕōĘųµÄĖŁĀŹ£»
£Ø2£©¢Ł°Ń¾Ę¾«µĘ³·×ߣ¬æŲÖĘŅ»¶ØµÄ¹ÄĘųĖŁ¶Č£¬ĶĖæÄܳ¤Ź±¼ä±£³ÖŗģČČÖ±µ½ŹµŃé½įŹų£¬ĖµĆ÷·“Ó¦Ņż·¢ŗ󣬲»Šč¼ÓČČ¼“æɽųŠŠµ½µ×£¬ĖµĆ÷øĆ·“Ó¦ŹĒ·ÅČȵķ“Ó¦£¬
¹Ź“š°øĪŖ£ŗ·ÅČČ£»
¢Ś¼×ÖŠµ„Ī»Ź±¼äÄŚµÄĘųÅŻŹżŌ½¶ą£¬ĘųĮ÷ĖŁ¶ČŌ½“󣬷“Ö®Ō½Š”£¬ĖłŅŌæÉŅŌøł¾Ż¼×ÖŠµ„Ī»Ź±¼äÄŚµÄĘųÅŻŹżĄ“æŲÖĘĘųĮ÷£¬
¹Ź“š°øĪŖ£ŗæŲÖĘ¼×ÖŠµ„Ī»Ź±¼äÄŚµÄĘųÅŻŹż£»
£Ø3£©ŅŅČ©Ņ×ČÜÓŚĖ®£¬ĪüŹÕŹ±Ó¦·ĄÖ¹µ¹ĪüµÄ·¢Éś£¬°²Č«ĘæÖŠµÄµ¼Ęų¹ÜŹĒ”°¶Ģ½ų³¤³ö”±£¬ĖłŅŌŅŅ½Ób£»
ŅŅČ©Ņ×»Ó·¢£¬²»ÓĆĖ®ĪüŹÕ¶ųŹĒÖ±½ÓĄäȓӦєŌń±łĖ®Ō”£¬
¹Ź“š°øĪŖ£ŗ·Ąµ¹Īü£»b£»±łĖ®£»
£Ø4£©ŅŅČ©ŗĶŠĀÖĘCu£ØOH£©2µÄ·“Ó¦·¢ÉśŃõ»Æ·“Ó¦£¬ŅŅČ©·Ö×ÓÖŠµÄČ©»ł±»Ńõ»ÆĪŖōČ»ł£¬ĒāŃõ»ÆĶ±»»¹ŌĪŖŃõ»ÆŃĒĶŗģÉ«³Įµķ£¬»Æѧ·½³ĢŹ½ĪŖCH3CHO+2Cu£ØOH£©2$\stackrel{¼ÓČČ}{”ś}$CH3COOH+Cu2O”ż+2H2O£¬
¹Ź“š°øĪŖ£ŗCH3CHO+2Cu£ØOH£©2$\stackrel{¼ÓČČ}{”ś}$CH3COOH+Cu2O”ż+2H2O£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅŅ“¼µÄ“ß»ÆŃõ»ÆŹµŃ飬ÕĘĪÕŅŅ“¼µÄ»ÆѧŠŌÖŹŅŌ¼°ŅŅ“¼µÄ“ß»ÆŃõ»ÆŹµŃé²Ł×÷Ź±½ā“šµÄ¹Ų¼ü£¬²ąÖŲæ¼²éѧɜ·ÖĪöĪŹĢā½āĢāĪŹĢāÄÜĮ¦£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆPtµē¼«µē½āÉŁĮæµÄMgCl2ČÜŅŗ£ŗ2H2O+2Cl-$\frac{\underline{\;µē½ā\;}}{\;}$H2”ü+Cl2”ü+2OH- | |
| B£® | H${\;}_{2}^{18}$OÖŠĶ¶ČėNa2O2¹ĢĢå£ŗ2H${\;}_{2}^{18}$O+2O${\;}_{2}^{2-}$ØT4OH-+18O2”ü | |
| C£® | Ca£ØHCO3£©2ČÜŅŗÓėÉŁĮæNaOHČÜŅŗ·“Ó¦£ŗHCO3-+Ca2++OH-ØTCaCO3”ż+H2O | |
| D£® | FeSO4ČÜŅŗÖŠ¼ÓH2O2ČÜŅŗ£ŗFe2++2H2O2+4H+ØTFe3++4H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 12”÷H3+5”÷H2-2”÷H1 | B£® | 2”÷H1-5”÷H2-12”÷H3 | C£® | 12”÷H3-5”÷H2-2”÷H1 | D£® | ”÷H1-5”÷H2-12”÷H3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼×ČÜŅŗÖŠŗ¬ÓŠHCO3- | B£® | ŅŅČÜŅŗÖŠŗ¬ÓŠSO42- | ||
| C£® | ±ūČÜŅŗÖŠŗ¬ÓŠMg2+ | D£® | ¶”ČÜŅŗÖŠŗ¬ÓŠNH4+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ü¢Ż¢Ž | B£® | ¢Ś¢Ū¢Ü¢Ż | C£® | ¢Ł¢Ū¢Ü¢Ż | D£® | ¢Ł¢Ś¢Ż¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½«Ļ”°±Ė®ÖšµĪ¼ÓČėĻ”ĮņĖįÖŠ£¬µ±ČÜŅŗµÄpH=7Ź±£¬c£ØSO42-£©£¾c£ØNH4+£© | |
| B£® | Į½ÖÖ“×ĖįČÜŅŗµÄĪļÖŹµÄĮæÅØ¶Č·Ö±šĪŖc1ŗĶc2£¬pHµÄ±šĪŖaŗĶa+1£¬Ōņc1=10c2 | |
| C£® | ³£ĪĀĻĀ£¬pH=11µÄNaOHČÜŅŗÓėpH=3µÄŃĪĖįČÜŅŗµČĢå»ż»ģŗĻ£¬µĪČėŹÆČļČÜŅŗ³ŹŗģÉ« | |
| D£® | Ļņ0.1mol•L-1µÄ°±Ė®ÖŠ¼ÓČėÉŁĮæĮņĖįļ§¹ĢĢ壬ŌņČÜŅŗÖŠc£ØOH-£©£ŗc£ØNH3•H2O£©¼õŠ” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | pH=5.6µÄCH3COOHÓėCH3COONa»ģŗĻČÜŅŗÖŠ£ŗc£ØNa+£©£¼c£ØCH3COO-£© | |
| B£® | ÅØ¶Č¾łĪŖ0.1mol--1LµÄCH3COOHŗĶCH3COONaČÜŅŗµČĢå»ż»ģŗĻŗó£ŗc£ØCH3COO-£©-c£ØCH3COOH£©=2[c£ØH+£©-c£ØOH-£©] | |
| C£® | ½«pH=aµÄ“×ĖįĻ”ŹĶĪŖpH=a+1µÄ¹ż³ĢÖŠ£¬$\frac{c£ØC{H}_{3}COOH£©}{c£Ø{H}^{+}£©}$²»±ä | |
| D£® | 0.2mol-L-1 CH3COOHČÜŅŗÖŠc£ØH+£©Óė 0.1mol-L-1 CH3COOHČÜŅŗÖŠc£ØH+£©µÄ±ČÖµŠ”ÓŚ2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | øß“æ¶ČµÄ¹č¹ć·ŗÓĆÓŚÖĘ×÷¹āµ¼ĻĖĪ¬£¬¹āµ¼ĻĖĪ¬ÓöĒæ¼ī»į”°¶ĻĀ·”± | |
| B£® | Ćŗ¾¹żĘų»ÆŗĶŅŗ»ÆĮ½øöĪļĄķ±ä»Æ£¬æɱäĪŖĒå½ąÄÜŌ“ | |
| C£® | Čē½«²ÄĮĻ¾łŌČ·ÖÉ¢µ½Ä³ŅŗĢå·ÖÉ¢¼ĮÖŠ£¬øĆ·ÖÉ¢ĻµæÉ·¢Éś¶”“ļ¶ūĻÖĻó£¬ÓÉ“ĖæÉĶĘ²āøĆ²ÄĮĻµÄÖ±¾¶ĪŖ1”«100pmÖ®¼ä | |
| D£® | ĶĄßĻßĻÅ®ŹæĄūÓĆŅŅĆŃŻĶČ”ĒąŻļĖŲ»ńµĆĮĖ2015Äź¶Čŵ±“¶ūÉśĄķѧ»ņŅ½Ń§½±£¬ĪŖČĖĄą·ĄÖĪű¼²×÷³öĮĖÖŲ“ó¹±Ļ× |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com