
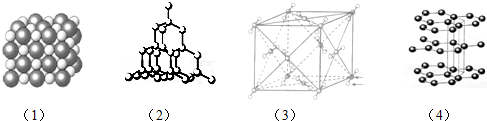
���� ��1��ͼ�У�1�������Ȼ��ƵĽṹͼ���Ȼ����ۻ�ʱ�ƻ����Ӽ���
��2��ͼ�У�2�����ڽ��ʯ�Ľṹͼ������ԭ�Ӿ��壻
��3��ͼ�У�3�����ڶ�����̼�Ľṹͼ��������̼���ڷ��Ӿ��壬�ۻ�ʱ�ƻ����Ӽ���������
��4��ͼ�У�4������ʯī�Ľṹͼ��ʯī���ڻ�Ͼ��壬���ʯ����ԭ�Ӿ��壻
��5���۵��һ����ɣ�ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壮
��� �⣺��1��ͼ�У�1�������Ȼ��ƵĽṹͼ���Ȼ����ۻ�ʱ�ƻ����Ӽ����������Ӽ��Ļ�����Ϊ��C��CaCl2��D��Ca��OH��2��E��NaF��
�ʴ�Ϊ��CDE��
��2��ͼ�У�2�����ڽ��ʯ�Ľṹͼ������ԭ�Ӿ��壬��������Ҳ����ԭ�Ӿ��壻
�ʴ�Ϊ��B��ԭ�ӣ�
��3��ͼ�У�3�����ڶ�����̼�Ľṹͼ��������̼���ڷ��Ӿ��壬�ۻ�ʱ�ƻ����Ӽ�������������Ҳ�Ƿ��Ӿ��壬�����ڷ��Ӿ������AF��
�ʴ�Ϊ��A�����Ӽ䣻
��4��ͼ�У�4������ʯī�Ľṹͼ��ʯī���ڻ�Ͼ��壬�����д��ڲ�״�ṹ�������֮���ܷ�������������ʯī��Ӳ�Ƚ�С�����ʯ����ԭ�Ӿ���Ӳ�Ⱥܴ�
�ʴ�Ϊ����2����
��5���۵��һ����ɣ�ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壬��2�����ڽ��ʯ�Ľṹͼ������ԭ�Ӿ��壬��1�������Ȼ��ƵĽṹͼ���Ȼ����������Ӿ��壬��3�����ڶ�����̼�Ľṹͼ��������̼���ڷ��Ӿ��壬���۵��ɸߵ��͵�����˳��Ϊ��2����1����3����
�ʴ�Ϊ����2����1����3����
���� ���⿼���˾������͵��жϡ���ѧ�����۵�Ƚϣ��ѶȲ����ݲ�ͬ���ʾ���Ľṹ�ص������ͼ������������������ɣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �϶����ڢ� | B�� | ���ٴ��ڢں͢� | C�� | ��ȷ���Ƿ��Т� | D�� | ���ٴ��ڢ١��ܡ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�ӵ�������������X��Y��Z | B�� | ���ʷе㣺Z��Y | ||
| C�� | ���Ӱ뾶��X2-��Y+��Z- | D�� | ԭ��������X��Y��Z |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
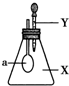 ��ͼ��ʾ����ƿ��ʢ������X���ι���ʢ��Һ��Y������ѹ��ͷ�ιܣ�ʹҺ��Y������ƿ�У�����һ������ɼ�С����a��������������X��Һ��Y�������ǣ�������
��ͼ��ʾ����ƿ��ʢ������X���ι���ʢ��Һ��Y������ѹ��ͷ�ιܣ�ʹҺ��Y������ƿ�У�����һ������ɼ�С����a��������������X��Һ��Y�������ǣ�������| X | Y | |
| A | NH3 | H2O |
| B | SO2 | NaOH��Һ |
| C | CO2 | 6mol•L-1 H2SO4��Һ |
| D | HCl | 6mol•L-1 Na2SO4��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��Ӧ������������ϵ������ͼ��ʾ | |
| B�� | ��H��ֵ�뷴Ӧ����ʽ�ļ���ϵ���й� | |
| C�� | �����÷�Ӧ��Ƴ�ԭ��أ�пΪ���� | |
| D�� | ���������Ϊԭ��أ�����32.5 gп�ܽ�ʱ�������ų�����һ��Ϊ11.2 L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| �� | ��1�� | ��2�� | ||||||
| �� | ��3�� | ��4�� | ��5�� | ��6�� | ��7�� | ��8�� | ��9�� | |
| �� | ��10�� | ��11�� | ��12�� |
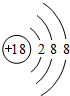 ��
��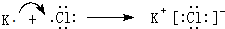 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ܷ���ȡ����Ӧ | B�� | �ܷ����ӳɷ�Ӧ | C�� | �ܷ����ۺϷ�Ӧ | D�� | ������ˮ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com