
��ѧ�����������е���ҵ���ڿ��Ź����У���ѧ�����Ų�����������á�
(1)��Ĺ�У�ʯ����(��Ҫ�ɷ�CaCO3)�ϻ��Ƶıڻ��ڳ�ʪ������������������һ���ɫ��Ĥ�����ⶨΪCaCO3���û�ѧ��Ӧ����ʽ��ʾ����CaCO3�Ĺ��̣�
(2)�Ż������е�Ǧ��[Pb2(OH)2CO3]�����ܿ�����������������ö�����ܽ�ȼ�С�ĺ�ɫ��Ǧ(��ӦI)��Ӱ�컭���ɫ���ù����������ʹ��Ǧ��ɰ�ɫ������(��Ӧ��)���ݴ˻ش��������⣺
��Ǧ����ǦԪ�صĻ��ϼ۳� �ۡ�
�ڷ�Ӧ�������ɵİ�ɫ���ʵĻ�ѧʽΪ ��
�۷�ӦI�Ļ�ѧ��Ӧ����ʽ ��
(3)�Ŵ�����(����ڵ���)������ȱ���Ļ����У���Ȼ��ʴ����(�绯��ʴ)��ԭ����һ�ֽ��������λ�ԭ����ϸ�������ṩ������Ӧ�Ĵ������ɽ������е�SO42����ԭΪS2-���÷�Ӧ�ų�����������ϸ����������ֳ֮�衣
��д���õ绯��ʴ��������Ӧ�ĵ缫��Ӧ ��
���������ǰ����������ĸ�ʴ���������(д��ѧʽ) ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
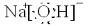
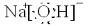
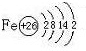
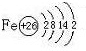
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
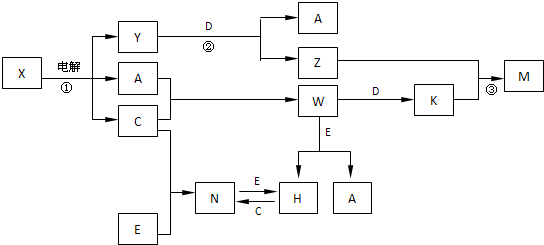
A��B��C��D��E����ѧ��ѧ�������ʣ��������ǵ�Ԫ�ص�ԭ�������ֱ�Ϊ����b�� c��d���֣���3 ��a+b�� = 2 ��a+c�� = 3 ��d-a����X��Y��Z��M��N��W��H��K�dz��������X��B��C�Ļ��ϲ������֮��������ת����ϵ��ͼ�з�Ӧ��Ͳ����е�H2O����ȥ����
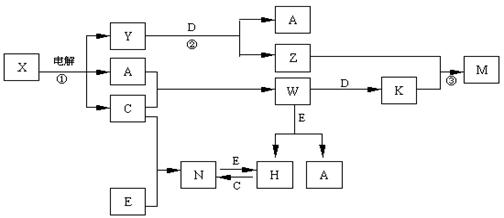
��1��Y�ĵ���ʽΪ ������D���ʵ�Ԫ�������ڱ���λ�� ���� �塣
��2����Ӧ�۵����ӷ���ʽΪ E����������D��һ�������·�Ӧ�Ļ�ѧ����ʽΪ�� ��
��3������N�����¼��ӷ���183�������������������е��л��ܼ���H2O�У��ݴ��ж�
NΪ �;��塣
��4��25��ʱ��PH��5��W��N��ˮ��Һ����H2O���������H������Ũ��֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�A��B��C��D��E����ѧ��ѧ�������ʣ��������ǵ�Ԫ�ص�ԭ�������ֱ�Ϊ����b�� c��d���֣���3 ��a+b�� = 2 ��a+c�� = 3 ��d-a����X��Y��Z��M��N��W��H��K�dz��������X��B��C�Ļ��ϲ������֮��������ת����ϵ��ͼ�з�Ӧ��Ͳ����е�H2O����ȥ����
��1��Y�ĵ���ʽΪ ������D���ʵ�Ԫ�������ڱ���λ�� ���� �塣
��2����Ӧ�۵����ӷ���ʽΪ E����������D��һ�������·�Ӧ�Ļ�ѧ����ʽΪ�� ��
��3������N�����¼��ӷ���183�������������������е��л��ܼ���H2O�У��ݴ��ж�
NΪ �;��塣
��4��25��ʱ��PH��5��W��N��ˮ��Һ����H2O���������H������Ũ��֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ӱ�ʡ2010�����һģģ�⣨�������ۻ�ѧ���� ���ͣ������
��12�֣�A��B��C��D��E����ѧ��ѧ�������ʣ��������ǵ�Ԫ�ص�ԭ�������ֱ�Ϊ����b�� c��d���֣���3 ��a+b�� = 2 ��a+c�� = 3 ��d-a����X��Y��Z��M��N��W��H��K�dz��������X��B��C�Ļ��ϲ������֮��������ת����ϵ��ͼ�з�Ӧ��Ͳ����е�H2O����ȥ����

��1��Y�ĵ���ʽΪ ������D���ʵ�Ԫ�������ڱ���λ�� ���� �塣
��2����Ӧ�۵����ӷ���ʽΪ E����������D��һ�������·�Ӧ�Ļ�ѧ����ʽΪ�� ��
��3������N�����¼��ӷ���183�������������������е��л��ܼ���H2O�У��ݴ��ж�
NΪ �;��塣
��4��25��ʱ��PH��5��W��N��ˮ��Һ����H2O���������H������Ũ��֮��Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com