
����8�֣�ij��ɫ��Һ�п��ܺ���Na����SO42�� ��MnO4����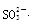 ��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
����pH��ֽ���飬��ҺpH��7��
������Һ�еμ���ˮ��������������ټ���CCl4������,CCl4��ʳȺ�ɫ���÷�Һ©����Һ��
�����Һ���ˮ��Һ�м���AgNO3��HNO3���Һ���а�ɫ����������
����ȡԭ��Һ��������Ba(NO3)2������Ļ��Һ��������ɫ������
�ش��������⣺
��1����Һ�п϶����е������� ���϶�û�е������� ��
��д��������е����ӷ���ʽ ��
����6�֣�SO2����ͨ����з�̪��NaOH��Һ����ɫ����ȥ�����ܵ�ԭ��
�� �� ��
�������ʵ��֤���� ��
����.��8�֣���Na+ ��Br����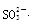
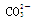 ��MnO4��
��MnO4��
��Cl2+2Br��== Br2+2 Cl�� 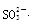 +Cl2+H2O ==2H++2Cl��+SO42��
+Cl2+H2O ==2H++2Cl��+SO42��
����.(6�֣�
�ټ���һ��SO2��SO2��ˮ���õIJ���Ư���˺�ɫ����Һ��
�ڼ������SO2��NaOH��Ӧ��������Һ��PH��������NaOH�����Լ������ɣ�
����һ��ȡ������ɫ�����Һ���Թܣ�����NaOH��Һ����Һ�ָ���ɫ��������������
��������ȡ������ɫ�����Һ���Թܣ��þƾ��Ƽ��ȣ���Һ�ָ���ɫ���������һ����
������������������������һ������һ��������жϣ������������£�
ij��ɫ��Һ�϶�û��MnO4������ɫ���п��ܺ���Na�����϶��У���Ϊֻ����һ�������ӣ���SO42�� ��MnO4���϶�û��MnO4������ɫ����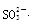 ��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
����pH��ֽ���飬��ҺpH��7��˵����Һ���ּ��ԣ������ڼ��Ի����в��ܴ�����������Ӳ��ܴ��ڣ��˴������ų��κ����ӣ�
������Һ�еμ���ˮ��������������ټ���CCl4������,CCl4��ʳȺ�ɫ���÷�Һ©����Һ��˵��һ����Br�����ӣ���Ϊ�嵥���������Ȼ�̼���dzȺ�ɫ�ģ�
�����Һ���ˮ��Һ�м���AgNO3��HNO3���Һ���а�ɫ�����������жϲ����Dz����������ӣ���Ϊǰ��ӵ�����ˮ�����к��������ӣ���ʵ�����и��ŵġ�ֻ��˵�����������ӡ�
����ȡԭ��Һ��������Ba(NO3)2������Ļ��Һ��������ɫ�������϶��������������Һ�е�������������Ӱ�����������������ˣ�����һ�������������������Һ�п϶����е�������Na+ ��Br����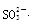
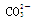 ���϶�û�е�������MnO4�� ��������е����ӷ���ʽ��Cl2+2Br��== Br2+2 Cl����
���϶�û�е�������MnO4�� ��������е����ӷ���ʽ��Cl2+2Br��== Br2+2 Cl����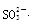 +Cl2+H2O ==2H++2Cl��+SO42����SO2����ͨ����з�̪��NaOH��Һ����ɫ����ȥ�����ܵ�ԭ��
+Cl2+H2O ==2H++2Cl��+SO42����SO2����ͨ����з�̪��NaOH��Һ����ɫ����ȥ�����ܵ�ԭ��
SO2��SO2��ˮ���õIJ���Ư���˺�ɫ����Һ��SO2��NaOH��Ӧ��������Һ��PH��
���ʵ��֤���ķ���������һ��ȡ������ɫ�����Һ���Թܣ�����NaOH��Һ����Һ�ָ���ɫ��������������
��������ȡ������ɫ�����Һ���Թܣ��þƾ��Ƽ��ȣ���Һ�ָ���ɫ���������һ������
���㣺��ѧʵ��
�����������ƶ���ⷨ���ɣ���Щ�ƶ���Ľⷨ�����������ӵ����з�Ӧ�Լ����Ӽ�Ĺ���������ڽ���֮ǰ��Ӧ�����ṩ����������Һ���ܷ����������з�������������������һ����˵�����Ӽ������ɳ����������塢��������ʣ��Լ��ܷ���������ԭ��Ӧ�ģ��Ͳ�������Һ�д������档���磬H+��OH- ��H+������������ӣ�OH- �����������ӣ�Ag+��Cl- ��Br- ��I- ��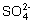 ��
��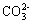 ��Ba2+��Ca2+��
��Ba2+��Ca2+��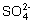 ��
��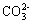 ��Fe2+��
��Fe2+��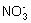 (����������)��Fe3+��S2- ��Al3+��
(����������)��Fe3+��S2- ��Al3+�� ��S2- �ȵȣ�����������Һ�й��档
��S2- �ȵȣ�����������Һ�й��档
�ھ����ƶϹ����У�Ҫע�����¼��㣺
(1) ���ƶ����ӵĿ϶�����ڽ���������ǣ���Ҫ����һ��
(2) �ƶϹ����У�ǰ��Ľ��۲�Ӧ��ì�ܡ���ˣ�ǰ�����½��۵����ӣ��ں�����ƶϹ����пɲ����������ڷ����з���ǰ�������ì�ܣ���Ӧ�ҳ�����ԭ��
(3) �����ƶϽ��ʱ��Ӧ�ÿ����������棬���϶����ڵ����ӣ��϶������ڵ����ӣ������ж������������ӡ��������������������Ӧ�ǻ�������ģ��κ�һ������ֻ�ܳ���һ�Σ������ظ����֡���Ȼ�е���Ŀ�в�һ�������������Ҫ�ش𣬵���������ʱ��Ӧ�ÿ��ǵ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ij��ɫ��Һ�пɴ����������������
A��Ba2+��NO3����SO32����Cl�� B��K+��Cl����SiO32����H+
C��K+��SO32����CO32����OH�� D��Cu2+��NO3����SO42����Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ij��ɫ��Һ�пɴ����������������
A��Ba2+��NO3����SO32����Cl�� B��K+��Cl����SiO32����H+
C��K+��SO32����CO32����OH�� D��Cu2+��NO3����SO42����Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ�����и߶�ѧҵˮƽ�����Ŀƻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ij��ɫ��Һ�пɴ����������������
A��Ba2+��NO3����SO32����Cl�� B��K+��Cl����SiO32����H+
C��K+��SO32����CO32����OH�� D��Cu2+��NO3����SO42����Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ�����и߶�ѧҵˮƽ�����Ŀƻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ij��ɫ��Һ�пɴ����������������
A��Ba2+��NO3����SO32����Cl�� B��K+��Cl����SiO32����H+
C��K+��SO32����CO32����OH�� D��Cu2+��NO3����SO42����Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ������������ѧ�߶�ѧҵˮƽ�����Ŀƻ�ѧ�Ծ����������� ���ͣ���ѡ��
��ij��ɫ��Һ�пɴ����������������
| A��Ba2+��NO3����SO32����Cl�� | B��K+��Cl����SiO32����H+ |
| C��K+��SO32����CO32����OH�� | D��Cu2+��NO3����SO42����Cl�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com