
![]() ��Һ�����ڸ�ʴӡˢ��·��,��Ӧ�����ӷ���ʽΪ ��
��Һ�����ڸ�ʴӡˢ��·��,��Ӧ�����ӷ���ʽΪ ��
(2)����1 mol ![]() ��2 mol
��2 mol ![]() �ĸ�ʴͭ���Ļ��Һ�м�������n mol,�Է�����Һ�������ӵĸ�����������±�:
�ĸ�ʴͭ���Ļ��Һ�м�������n mol,�Է�����Һ�������ӵĸ�����������±�:
| n��ȡֵ��Χ | ��Һ�������� |
| n<1 | |
|
| |
| n |
(3)��ʴͭ���Ļ��Һ��,��![]() ��
��![]() ��
��![]() ��Ũ�Ⱦ�Ϊ0.10
��Ũ�Ⱦ�Ϊ0.10 ![]() ������±����������ݺ�ҩƷ,������ȥ
������±����������ݺ�ҩƷ,������ȥ![]() ��Һ��
��Һ��![]() ��
��![]() ��ʵ�鲽��: ��
��ʵ�鲽��: ��
| �������↑ʼ����ʱ��pH | �������������ȫʱ��pH | |
| Fe3+ | 1.9 | 3.2 |
| Fe2+ | 7.0 | 9.0 |
| Cu2+ | 4.7 | 6.7 |
| �ṩ��ҩƷ: | ||

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������������������һ��ѧ��ѧҵ���г�����ۻ�ѧ�Ծ��������棩 ���ͣ������
��Ԫ�ؼ��仯�������������������ϢϢ��أ��Իش��������⣺
��1�����ӹ�ҵ����30����FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�壬�÷�Ӧ�����ӷ���ʽΪ ��
��2����֪��Fe(s)+ O2(g)
O2(g) FeO(s)
��H=��272 kJ��mol��1
FeO(s)
��H=��272 kJ��mol��1
C(s)+O2(g) CO2(g) ��H=��393.5 kJ��mol��1
CO2(g) ��H=��393.5 kJ��mol��1
2C(s)+O2(g) 2CO(g) ��H=��221 kJ��mol��1
2CO(g) ��H=��221 kJ��mol��1
���¯����������
FeO(s)+CO(g) Fe(s)+CO2(g) ��H=
��
Fe(s)+CO2(g) ��H=
��
��3�����죨Fe2O3����һ�ֺ�ɫ���ϡ���һ��������������160mL 5 mol��L��1�����У��ټ����������ۣ�����Ӧ�������ռ�������2.24L����״�������������Һ����Fe3������μӷ�Ӧ�����۵�����Ϊ ��
��4����H2��O2��������Na2CO3���ȼ�ϵ�أ����õ�ⷨ�Ʊ�Fe(OH)2��װ������ͼ��ʾ������P��ͨ��CO2��
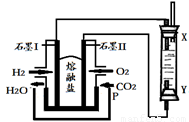
��ʯīI�缫�ϵĵ缫��ӦʽΪ ��
��ͨ��һ��ʱ����Ҳಣ�����в��������İ�ɫ�������ҽϳ�ʱ�䲻��ɫ��������˵������ȷ���� ������ţ���
A��X��Y���˶������������缫
B��������NaOH��Һ��Ϊ���Һ
C�����������ķ�Ӧ�ǣ�2H2O�� 2e��= H2��+ 2OH��
D����ɫ����ֻ���������ϲ���
����������Fe(OH)2������¶�ڿ����У�����ɫ�仯Ϊ ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧѡ��2 4.1 ���ϵļӹ�������ϰ���������棩 ���ͣ������
�������仯�����Ӧ�úܹ㷺���־ټ��ֳ����������仯�������;���ش��й����⣺
(1)FeCl3��Һ�����ڸ�ʴӡˢ��·ͭ�壬�����ӷ���ʽΪ��_______________________________��
(2)��ͭ��������õ��Ĵ�ͭ�к�����Fe��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ơ��������ͭ���õ���ͭ��ԭ����_________________________________________��
(3)ij������Ա�������ʲ���������и�ʴ����������ijЩ����Һ�и�ʴ�������ԡ�����±��ṩ��ҩƷ��ѡ������(ˮ����ѡ)��������ʵ�飬��֤���ʲ�����ױ���ʴ��
|
Ũ���ᡢϡ���ᡢCuO��NaOH��Һ |
�йط�Ӧ�Ļ�ѧ����ʽ____________________________________________________��
���ʲ���ָ�ʴ��ʵ������________________________________________________��
(4)�Ͻ����������������;Խ��Խ�㷺��������ص�֪ʶ�����ں���ҵ�����Ͻ�����Ͻ���;���㷺��ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ũ���ᡢϡ���ᡢCuO��NaOH��Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com