
ķŚ£ØTe£©ĪŖ¢öA×åŌŖĖŲ£¬ŹĒµ±½ńøߊĀ¼¼ŹõŠĀ²ÄĮĻµÄÖ÷ŅŖ³É·ÖÖ®Ņ»”£¹¤ŅµÉĻæÉ“Óµē½ā¾«Į¶ĶµÄŃō¼«ÄąÖŠĢįČ”ķŚ”£
£Ø1£©“ÖĶÖŠŗ¬ÓŠCuŗĶÉŁĮæZn”¢Ag”¢Au”¢TeO2¼°ĘäĖū»ÆŗĻĪļ£¬µē½ā¾«Į¶ŗó£¬Ńō¼«ÄąÖŠÖ÷ŅŖŗ¬ÓŠTeO2”¢ÉŁĮ潚Źōµ„ÖŹ¼°ĘäĖū»ÆŗĻĪļ”£µē½ā¾«Į¶“ÖĶŹ±£¬Ńō¼«µē¼«·“Ó¦Ź½ĪŖ____________”£
£Ø2£©TeO2ŹĒĮ½ŠŌŃõ»ÆĪļ£¬Ī¢ČÜÓŚĖ®£¬æÉČÜÓŚĒæĖį»ņĒæ¼ī”£“ÓÉĻŹöŃō¼«ÄąÖŠĢįČ”ķŚµÄŅ»ÖÖ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
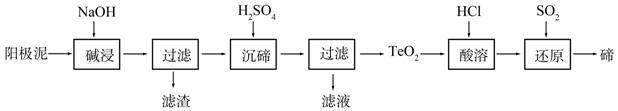
¢Ł”°¼ī½ž”±Ź±TeO2·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________________”£
¢Ś”°³ĮķŚ”±Ź±æŲÖĘČÜŅŗµÄpHĪŖ4.5-5.0£¬Éś³ÉTeO2³Įµķ”£Čē¹ūH2SO4¹żĮ棬ČÜŅŗĖį¶Č¹ż“󣬽«µ¼ÖĀķŚµÄ³Įµķ
²»ĶźČ«£¬ŌŅņŹĒ____________________________________£»·ĄÖ¹¾Ö²æĖį¶Č¹ż“óµÄ²Ł×÷·½·ØŹĒ_______________________________________________________________”£
¢Ū”°ĖįČÜ”±ŗ󣬽«SO2ĶØČėTeCl4ČÜŅŗÖŠ½ųŠŠ”°»¹Ō”±µĆµ½ķŚ£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ
______________________________________________________ӣ

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¢Čż·ś»ÆµŖ(NF3)ŹĒĪŽÉ«ĪŽĪ¶ĘųĢ壬ĖüæÉÓÉ°±ĘųŗĶ·śĘų·“Ó¦ÖĘµĆ£ŗ4NH3£«3F2===NF3£«3NH4F”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A”¢NH4FµÄ»¹ŌŠŌ±ČNH3Ēæ
B”¢NF3µÄŃõ»ÆŠŌ±ČF2Ēæ
C”¢øĆ·“Ó¦ÖŠ±»Ńõ»ÆŗĶ±»»¹ŌĪļÖŹµÄĪļÖŹµÄĮæÖ®±ČĪŖ4”Ć3
D”¢øĆ·“Ó¦ÖŠµĆµ½1 mol NF3Ź±£¬×ŖŅĘ6 molµē×Ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
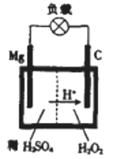
1808Äź£¬Ó¢¹ś»Æѧ¼ŅÓĆ¼Ų»¹ŌŃõ»ÆĆ¾£¬×īŌēÖʵĆÉŁĮæµÄĆ¾”£Ć¾ŹĒŗ½æÕ¹¤ŅµµÄÖŲŅŖ²ÄĮĻ£¬Ć¾×÷ĪŖŅ»ÖÖĒæŃõ»Æ¼Į£¬»¹ÓĆÓŚīŃ”¢īėŗĶÓĖµÄÉś²śÖŠ”£
£Ø1£©Ć¾ŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ_________________________.
(2)Š“³öÓĆĆ¾Óė½šŗģŹÆ£ØÖ÷ŅŖ³É·ÖĪŖTi O2£©ŌŚ¼ÓČČĢõ¼žĻĀÖĘČ”īѵĻÆѧ·½³ĢŹ½£ŗ_____________________________________.
(3)Ć¾ŌŚ¼ÓČȵÄĢõ¼žĻĀ»¹ÄÜÓėNaOH¹ĢĢå·“Ó¦£¬Éś³ÉMgOŗĶµ„ÖŹX”¢µ„ÖŹY”£ŅŃÖŖXÓėĖ®·“Ó¦æÉÉś³Éµ„ÖŹY£¬ŌņĆ¾ÓėNaOH·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________________.
(4)Ć¾—¹żŃõ»ÆĒāµē³ŲµÄ¹¤×÷ŌĄķČēĶ¼ĖłŹ¾£¬øƵē³Ų·ÅµēŹ±×Ü·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______________________________________.
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓÉĀĮĶĮæó(Ö÷ŅŖ³É·ÖŹĒAl2O3)Į¶ÖĘĀĮµÄ¹¤ŅÕĮ÷³ĢŹ¾ŅāĶ¼ČēĻĀ:

(1)µē½āÉś³ÉµÄĀĮŌŚČŪČŚŅŗµÄ””””(Ģī”°ÉĻ²ć”±»ņ”°ĻĀ²ć”±),µē½āŹ±²»¶ĻĻūŗĵĵē¼«ŹĒ””””(Ģī”°Ņõ¼«”±»ņ”°Ńō¼«”±)”£
(2)Š“³öĶØČė¹żĮ涞Ńõ»ÆĢ¼Ėį»ÆŹ±·“Ó¦µÄĄė×Ó·½³ĢŹ½:”””””””””””””””””””””””””””””””””””””””””£
(3)µē½āÖĘ ±øĀĮŹ±,Šč¼ÓČė±ł¾§ŹÆ(Na3AlF6),Ęä×÷
±øĀĮŹ±,Šč¼ÓČė±ł¾§ŹÆ(Na3AlF6),Ęä×÷ ÓĆŹĒ””””””””””””””””””””””,¹¤ŅµÉĻæÉŅŌÓĆ·ś»ÆĒāĘųĢ唢ĒāŃõ»ÆĀĮŗĶ“æ¼īŌŚøßĪĀĢõ¼žĻĀ·¢Éś·“Ó¦Ą“ÖĘČ”±ł¾§ŹÆ,Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½:”””””””””””””””””””””£
ÓĆŹĒ””””””””””””””””””””””,¹¤ŅµÉĻæÉŅŌÓĆ·ś»ÆĒāĘųĢ唢ĒāŃõ»ÆĀĮŗĶ“æ¼īŌŚøßĪĀĢõ¼žĻĀ·¢Éś·“Ó¦Ą“ÖĘČ”±ł¾§ŹÆ,Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½:”””””””””””””””””””””£
(4)ÉĻŹö¹¤ŅÕĖłµĆĀĮÖŠĶłĶłŗ¬ÓŠÉŁĮæFeŗĶSiµČŌÓÖŹ,æÉÓƵē½ā·½·Ø½ųŅ»²½Ģį“æ,øƵē½ā³ŲµÄŅõ¼«²ÄĮĻŹĒ””””(Ģī»ÆѧŹ½),Ńō¼«µÄµē¼«·“Ó¦ĪŖ”””””””””””””””””””” ”””””£
”””””£
(5)¶Ō½šŹōÖĘĘ·½ųŠŠæ¹øÆŹ““¦Ąķ,æÉŃÓ³¤ĘäŹ¹ÓĆŹŁĆü”£
¢ŁæŲÖĘŅ»¶ØĢõ¼ž½ųŠŠµē½ā(¼ūĻĀĶ¼),“ĖŹ±ĀĮ±ķĆęæÉŠĪ³ÉÄĶĖįµÄÖĀĆÜŃõ»ÆĤ,Ęäµē¼«·“Ó¦ĪŖ””””””””””;

¢ŚøֲĶĘĀĮŗó,ÄÜ·ĄÖ¹øÖ²ÄøÆŹ“,ĘäŌŅņŹĒ”””””””””””””””””””””””””””””””””””””””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
·Ö×ÓŹ½ĪŖC10H20O2µÄÓŠ»śĪļŌŚĖįŠŌĢõ¼žĻĀæÉĖ®½āĪŖ“¼AŗĶĖįB£¬A¾¹żĮ¬ŠųŃõ»ÆæÉ×Ŗ»ÆĪŖB£¬Čō²»æ¼ĀĒĮ¢ĢåŅģ¹¹£¬·ūŗĻÉĻŹöŅŖĒóµÄ“¼ŗĶĖįČōÖŲŠĀ×éŗĻ£¬æÉŠĪ³ÉµÄõ„¹²ÓŠ£ŗ£Ø £©
A£®32 B£®16 C£®8 D£®4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖĪ¬ÉśĖŲAµÄ½į¹¹¼ņŹ½ČēĶ¼ĖłŹ¾£¬¹ŲÓŚĖüµÄĖµ·ØÕżČ·µÄŹĒ(””””)

A£®Ī¬ÉśĖŲAŹĒŅ»ÖÖ·Ó
B£®Ī¬ÉśĖŲA¾ßÓŠ»·¼ŗĶéµÄ½į¹¹µ„ŌŖ
C£®Ī¬ÉśĖŲAµÄŅ»øö·Ö×ÓÖŠÓŠ3øöĖ«¼ü
D£®Ī¬ÉśĖŲAµÄŅ»øö·Ö×ÓÖŠÓŠ30øöĒāŌ×Ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
·Ö×ÓŹ½ĪŖC2H4O2µÄ½į¹¹æÉÄÜÓŠ ŗĶ
ŗĶ Į½ÖÖ£¬ĪŖ¶ŌĘä½į¹¹½ųŠŠĪļĄķ·½·Ø¼ų¶Ø£¬æÉÓĆ______________»ņ________________”£
Į½ÖÖ£¬ĪŖ¶ŌĘä½į¹¹½ųŠŠĪļĄķ·½·Ø¼ų¶Ø£¬æÉÓĆ______________»ņ________________”£
(1)ČōĪŖ £¬ŌņŗģĶā¹āĘ×ÖŠÓ¦øĆÓŠ____øöÕń¶ÆĪüŹÕ£»ŗĖ“Ź²ÕńĒāĘ×ÖŠÓ¦øĆÓŠ__________øö·å”£
£¬ŌņŗģĶā¹āĘ×ÖŠÓ¦øĆÓŠ____øöÕń¶ÆĪüŹÕ£»ŗĖ“Ź²ÕńĒāĘ×ÖŠÓ¦øĆÓŠ__________øö·å”£
(2)ČōĪŖ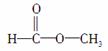 £¬ŌņŗģĶā¹āĘ×ÖŠÓŠ______øöÕń¶ÆĪüŹÕ£»ŗĖ“Ź²ÕńĒāĘ×ÖŠÓ¦ÓŠ______øö·å”£
£¬ŌņŗģĶā¹āĘ×ÖŠÓŠ______øöÕń¶ÆĪüŹÕ£»ŗĖ“Ź²ÕńĒāĘ×ÖŠÓ¦ÓŠ______øö·å”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŃĒĀČĖįÄĘ£ØNaClO2£©ŹĒŅ»ÖÖÖŲŅŖµÄŗ¬ĀČĻū¶¾¼Į£¬³£ÓĆÓŚĖ®µÄĻū¶¾ŅŌ¼°·ÄÖÆøßĘÆ°×”£¹żŃõ»ÆĒā·ØÉś²śŃĒĀČĖįÄʵÄĮ÷³ĢĶ¼ČēĻĀ
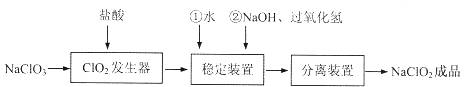
ŅŃÖŖNaClO2µÄČܽā¶ČĖęĪĀ¶ČÉżø߶ųŌö“ó£¬ŹŹµ±Ģõ¼žĻĀæɽį¾§Īö³öNaClO2·3H2OĒŅNaClO2ŌŚ¼īŠŌĢõ¼žĻĀĪČ¶ØŠŌ½Ļøß”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŚClO2·¢ÉśĘ÷ÖŠĶ¬Ź±ÓŠĀČĘų²śÉś£¬ŌņŌŚ·¢ÉśĘ÷ÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£Ø2£©ŌŚNaClO2ĪȶØ×°ÖĆÖŠ£¬H2O2×÷ £ØŃ”ĢīŠņŗÅ£©”£
A£®Ńõ»Æ¼Į B£®»¹Ō¼Į
C£®¼Č×÷Ńõ»Æ¼ĮÓÖ×÷»¹Ō¼Į D£®¼Č²»×÷Ńõ»Æ¼ĮŅ²²»×÷»¹Ō¼Į
£Ø3£©ŌŚŹµŃéŹŅÄ£Äā”°¹ĢĢå·ÖĄė×°ÖĆ”±ÖŠµÄ¼¼Źõ£¬±ŲŠė½ųŠŠµÄŹµŃé²Ł×÷ŹĒ £Ø°“ŹµŃéŗóĖ³ŠņĢīŠ“²Ł×÷“śŗÅ£©”£
A£®¹żĀĖ B£®¼ÓČČ C£®·ÖŅŗ D£®ÕōĮó E£®ĄäČ“
£Ø4£©¾²éŌÄ׏ĮĻÖŖµĄ£ŗµ±pH”Ü2.0Ź±£¬ClO-2Äܱ»IĶźČ«»¹Ō³ÉCl—£»
ČÜŅŗÖŠNa2S2O3ÄÜÓėI2·“Ӧɜ³ÉNaIŗĶNa2S4O6”£
Óū²ā¶Ø³ÉĘ·ÖŠNaClO2µÄŗ¬Į棬ĻÖ½ųŠŠČēĻĀ²Ł×÷£ŗ

¢Ł²½Öč¢ņÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ £¬
²½Öč¢óÖŠ“ļµ½µĪ¶ØÖÕµćŹ±µÄĻÖĻóŹĒ ”£
¢ŚČōÉĻŹöµĪ¶Ø²Ł×÷ÖŠÓĆČ„ĮĖV mL Na2S2O3ČÜŅŗ£¬Ōņѳʷ֊NaClO2µÄÖŹĮæ·ÖŹż £ØÓĆ×ÖÄø±ķŹ¾£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£®ŅŃÖŖHCN(aq)ÓėNaOH(aq)·“Ӧɜ³É1 molÕżŃĪµÄ¦¤H£½£12.1 kJ/mol£»ĒæĖį”¢Ēæ¼īµÄĻ”ČÜŅŗ·“Ó¦µÄÖŠŗĶČȦ¤H£½£57.3 kJ·mol£1”£ŌņHCNŌŚĖ®ČÜŅŗÖŠµēĄėµÄ¦¤HµČÓŚ
A£®£69.4 kJ·mol£1 B£®£45.2 kJ·mol£1
C£®£«69.4 kJ·mol£1 D£®£«45.2 kJ·mol£1
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com