
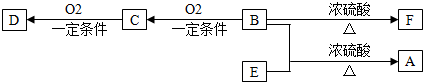
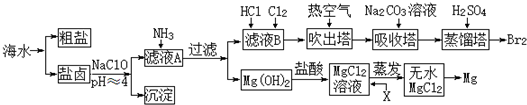
���� �л���A��C10H20O2����������ζ��������������ϴ���㲨�ķ��㸳�������A��������B����������������B�Ǵ���E�����ᣬB��û��֧����D����̼�����Ʒ�Ӧ����D�����ᣬD��E��Ϊ������ͬ�����ŵ�ͬ���칹����B��E��̼ԭ�Ӹ�����ȣ�����B��E������5��̼ԭ�ӣ�B��������������B�д��ǻ�λ�ڱ��ϣ���B�ṹ��ʽΪCH3��CH2��3CH2OH��CΪCH3��CH2��3CHO��DΪCH3��CH2��3COOH��E���������ϵ���������ԭ��ȡ������һ�ȴ���ֻ��һ��˵��E��ֻ��һ����ԭ�ӣ���E�ṹ��ʽΪ��CH3��3CCOOH��F����ʹ������Ȼ�̼��Һ��ɫ����B������ȥ��Ӧ����F��F�к���̼̼˫������F�ṹ��ʽΪCH3��CH2��2CCH=CH2���ݴ˷������
��� �⣺�л���A��C10H20O2����������ζ��������������ϴ���㲨�ķ��㸳�������A��������B����������������B�Ǵ���E�����ᣬB��û��֧����D����̼�����Ʒ�Ӧ����D�����ᣬD��E��Ϊ������ͬ�����ŵ�ͬ���칹����B��E��̼ԭ�Ӹ�����ȣ�����B��E������5��̼ԭ�ӣ�B��������������B�д��ǻ�λ�ڱ��ϣ���B�ṹ��ʽΪCH3��CH2��3CH2OH��CΪCH3��CH2��3CHO��DΪCH3��CH2��3COOH��E���������ϵ���������ԭ��ȡ������һ�ȴ���ֻ��һ��˵��E��ֻ��һ����ԭ�ӣ���E�ṹ��ʽΪ��CH3��3CCOOH��F����ʹ������Ȼ�̼��Һ��ɫ����B������ȥ��Ӧ����F��F�к���̼̼˫������F�ṹ��ʽΪCH3��CH2��2CCH=CH2��
��1��BΪCH3��CH2��3CH2OH��B�к��д��ǻ����ܷ���ȡ����Ӧ��������Ӧ����ȥ��Ӧ��������Ӧ����ѡ�٢ڢܣ�
��2��DΪCH3��CH2��3COOH��F�ṹ��ʽΪCH3��CH2��2CCH=CH2��D��F�й��������Ʒֱ����Ȼ���̼̼˫����
�ʴ�Ϊ���Ȼ���̼̼˫����
��3����D��E������ͬ�����ŵ�ͬ���칹��Ŀ��ܽṹ��ʽ����CH3��2CHCH2COOH��CH3CH2CH��CH3��COOH��
�ʴ�Ϊ����CH3��2CHCH2COOH��CH3CH2CH��CH3��COOH��
��4��BΪCH3��CH2��3CH2OH��FΪCH3��CH2��2CCH=CH2��B������ȥ��Ӧ����F����Ӧ����ʽΪ��CH3CH2CH2CH2CH2OH$��_{��}^{Ũ����}$CH3CH2CH2CH=CH2+H2O��
�ʴ�Ϊ��CH3CH2CH2CH2CH2OH$��_{��}^{Ũ����}$CH3CH2CH2CH=CH2+H2O��
��5��E�ṹ��ʽΪ��CH3��3CCOOH��E��2-��-1-�����ͼ�����һ����������ȡE���÷�Ӧ�Ļ�ѧ����ʽ�ǣ���CH3��2CHCH2OH+HCOOH$\stackrel{һ��������}{��}$��CH3��3CCOOH+H2O��
�ʴ�Ϊ����CH3��2CHCH2OH+HCOOH$\stackrel{һ��������}{��}$��CH3��3CCOOH+H2O��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶϼ���ȡ��Ϣ��������Ϣ����������A����Ϣ���������Ƶ����������ƶϣ��漰����ȩ�����ᡢ����ϩ��֮���ת�����ѵ��ǣ�5���ⷽ��ʽ����д��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��һ���¿�����ѧ�Ծ��������棩 ���ͣ�ѡ����
��3 mol��L��1��Ca(NO3)2��Һa mLϡ����b mL��ϡ�ͺ���Һ��NO �����ʵ���Ũ��Ϊ�� ��
�����ʵ���Ũ��Ϊ�� ��
A��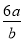 mol��L��1 B��
mol��L��1 B��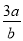 mol��L��1 C��
mol��L��1 C�� mol��L��1 D��
mol��L��1 D��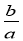 mol��L��1
mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢ� | B�� | �٢ܢ� | C�� | �٢ڢ� | D�� | �ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol•L-1��Na3PO4��Һ�У�c��Na+��=3c��PO43-��+2c��HPO42-��+c��H2PO4-�� | |
| B�� | 0.1mol•L-1��NaHS��Һ�У�c��H+��+c��H2S��=c��OH-��+c��S2-�� | |
| C�� | 0.1mol•L-1�İ�ˮ��0.1mol•L-1��NaHSO4��Һ�������ϣ�c��Na+ ��=c��SO42-��=c��NH4+����C��H+����C��OH-�� | |
| D�� | 0.1mol•L-1��Na2CO3��Һ��0.1mol•L-1��NaHCO3��Һ�������ϣ�c��Na+����c��CO32-����c��HCO3-����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| R | X | |
| T | ||
| Z | Q |
| A�� | R�ĵ��ʽṹʽΪ�� | |
| B�� | ��ҵ���Ժ�ˮΪԭ�ϣ���������ԭ��Ӧ�Ʊ�Q�ĵ��� | |
| C�� | �ǽ����ԣ�Z��T��X | |
| D�� | Rԭ����Zԭ�ӵĵ��������26 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��R1��R2��R3������������ԭ�ӣ�
��R1��R2��R3������������ԭ�ӣ� ��
�� ��
�� ����һ��ȡ��������E�����������ţ���ʹ��˳������Լ�ΪNa��������Һ�������Ƶ�Cu��OH��2����Һ����
����һ��ȡ��������E�����������ţ���ʹ��˳������Լ�ΪNa��������Һ�������Ƶ�Cu��OH��2����Һ����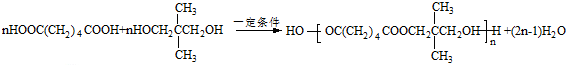 ��
��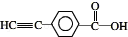 ��
�� ��
��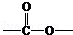 ����
���� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��̬�⻯��ˮ��Һ���������μ��� | |
| B�� | ��ˮ����ˮ����ˮ����ʹ���۵⻯����ֽ���� | |
| C�� | ��̬�⻯����ȶ�����ԭ���������������ǿ | |
| D�� | ԭ�Ӻ���������������ԭ����������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʵʩ����ȼ�ϡ������������������������������͵�����������Ⱦ | |
| B�� | ����������������г��õ�������Ѿ���һ���������SO2��Ϊ���Ӽ� | |
| C�� | ��֬�����ۡ���ά�ء������ʵȶ�����������ˮ�Ⲣ�ṩ���� | |
| D�� | ���������������Լ����������������������������Ҫ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com