
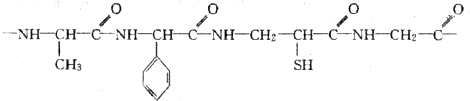
 ЖЯЬМЕЊЕЅМќЃЌЬМЩЯМг-OHЃЌЕЊЩЯМг-HЃЌЫЎНтЕУЕНЕФАБЛљЫсЮЊCH3CHЃЈNH2ЃЉCOOHЁЂH2NCH2COOHЁЂH2NCH2CHЃЈSHЃЉCOOHЁЂH2NCHЃЈC6H5ЃЉCOOHЃЌдђH2NCHЃЈC6H5ЃЉCOOHжаCЁЂHдзгИіЪ§БШзюДѓЃЛ
ЖЯЬМЕЊЕЅМќЃЌЬМЩЯМг-OHЃЌЕЊЩЯМг-HЃЌЫЎНтЕУЕНЕФАБЛљЫсЮЊCH3CHЃЈNH2ЃЉCOOHЁЂH2NCH2COOHЁЂH2NCH2CHЃЈSHЃЉCOOHЁЂH2NCHЃЈC6H5ЃЉCOOHЃЌдђH2NCHЃЈC6H5ЃЉCOOHжаCЁЂHдзгИіЪ§БШзюДѓЃЛ ЃЌЫЎНташвЊ4ИіЫЎЗжзгЩњГЩАБЛљЫсЃЎ
ЃЌЫЎНташвЊ4ИіЫЎЗжзгЩњГЩАБЛљЫсЃЎ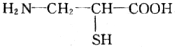 ЃЌЙЪД№АИЮЊЃК
ЃЌЙЪД№АИЮЊЃК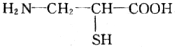 ЃЛ
ЃЛ ЖЯЬМЕЊЕЅМќЃЌЬМЩЯМг-OHЃЌЕЊЩЯМг-HЃЌЫЎНтЕУЕНЕФАБЛљЫсЮЊCH3CHЃЈNH2ЃЉCOOHЁЂH2NCH2COOHЁЂH2NCH2CHЃЈSHЃЉCOOHЁЂH2NCHЃЈC6H5ЃЉCOOHЃЌдђH2NCHЃЈC6H5ЃЉCOOHжаCЁЂHдзгИіЪ§БШзюДѓЃЌ
ЖЯЬМЕЊЕЅМќЃЌЬМЩЯМг-OHЃЌЕЊЩЯМг-HЃЌЫЎНтЕУЕНЕФАБЛљЫсЮЊCH3CHЃЈNH2ЃЉCOOHЁЂH2NCH2COOHЁЂH2NCH2CHЃЈSHЃЉCOOHЁЂH2NCHЃЈC6H5ЃЉCOOHЃЌдђH2NCHЃЈC6H5ЃЉCOOHжаCЁЂHдзгИіЪ§БШзюДѓЃЌ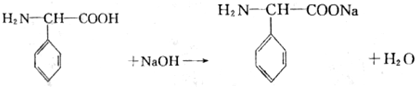 ЃЌ
ЃЌ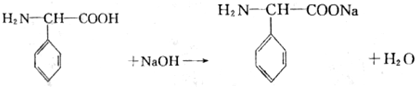 ЃЛ
ЃЛ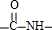 ЃЌЫЎНташвЊ4ИіЫЎЗжзгЩњГЩАБЛљЫсдђЫЎНтЩњГЩЕФИїжжАБЛљЫсЕФЪНСПжЎКЭЮЊ364+18ЁС4=436ЃЌЙЪД№АИЮЊЃК436ЃЎ
ЃЌЫЎНташвЊ4ИіЫЎЗжзгЩњГЩАБЛљЫсдђЫЎНтЩњГЩЕФИїжжАБЛљЫсЕФЪНСПжЎКЭЮЊ364+18ЁС4=436ЃЌЙЪД№АИЮЊЃК436ЃЎ


 УћаЃПЮЬУЯЕСаД№АИ
УћаЃПЮЬУЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| AЁЂЙшЪЧЗЧН№ЪєдЊЫиЃЌЕЋЫќЕФЕЅжЪЪЧЛвКкЩЋгаН№ЪєЙтдѓЕФЙЬЬх |
| BЁЂЕБМгШШЕНвЛЖЈГЬЖШЪБЃЌЙшФмгыбѕЦјЁЂЧтЦјЕШЗЧН№ЪєЗДгІ |
| CЁЂЖўбѕЛЏЙшМШЪЧЫсадбѕЛЏЮяЃЌгжЪЧМюадбѕЛЏЮя |
| DЁЂЙшЕФЕМЕчадФмНщгкН№ЪєКЭОјдЕЬхжЎМфЃЌЪЧСМКУЕФАыЕМЬхВФСЯ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
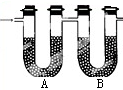 вбжЊФГжжЦјЬЌПѓЮяШМСЯКЌгаЬМКЭЧтСНжждЊЫиЃЎЮЊСЫВтЖЈетжжШМСЯжаЬМКЭЧтСНжждЊЫиЕФжЪСПБШЃЌПЩНЋЦјЬЌШМСЯЗХШызуСПЕФбѕЦјжаШМЩеЃЌВЂЪЙВњЩњЕФЦјЬхШЋВПЭЈШыЭМЫљЪОЕФзАжУЃЈЦјЬхСїЯђШчЭМЃЉЃЌЕУЕНШчЯТБэЫљСаЕФЪЕбщНсЙћЃЈМйЩшВњЩњЕФЦјЬхЭъШЋБЛЮќЪеЃЉЃЎ
вбжЊФГжжЦјЬЌПѓЮяШМСЯКЌгаЬМКЭЧтСНжждЊЫиЃЎЮЊСЫВтЖЈетжжШМСЯжаЬМКЭЧтСНжждЊЫиЕФжЪСПБШЃЌПЩНЋЦјЬЌШМСЯЗХШызуСПЕФбѕЦјжаШМЩеЃЌВЂЪЙВњЩњЕФЦјЬхШЋВПЭЈШыЭМЫљЪОЕФзАжУЃЈЦјЬхСїЯђШчЭМЃЉЃЌЕУЕНШчЯТБэЫљСаЕФЪЕбщНсЙћЃЈМйЩшВњЩњЕФЦјЬхЭъШЋБЛЮќЪеЃЉЃЎ| зАжУЕФжЪСП | ЪЕбщЧА | ЪЕбщКѓ |
| ЃЈЙЬЬх+UаЮЙмAЃЉ | 101.1g | 102.9g |
| ЃЈЙЬЬх+UаЮЙмBЃЉ | 312.0g | 314.2g |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| AЁЂЛ№аЧЫФЩф | BЁЂЗЂШШ |
| CЁЂЩњГЩКкЩЋЙЬЬх | DЁЂЙЬЬхаЮзДБфЛЏСЫ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| AЁЂШегУТСжЦЦЗБэУцИВИЧзХбѕЛЏФЄЃЌЖдФкВПН№ЪєЦ№БЃЛЄзїгУ |
| BЁЂЕЊЕФЙЬЖЈжЛгадкИпЮТЁЂИпбЙЁЂДпЛЏМСЕФЬѕМўЯТВХФмЪЕЯж |
| CЁЂЙшНКЪшЫЩЖрПзЃЌПЩгУзїДпЛЏМСЕФдиЬх |
| DЁЂИпДПЖШЕФЙшЕЅжЪЙуЗКгУгкжЦзїЙтЕМЯЫЮЌ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| AЁЂ53ПЫNa2CO3ШмНтдк947ПЫЫЎжа |
| BЁЂ53ПЫNa2CO3ШмНтдк1LЫЎжа |
| CЁЂ53ПЫNa2CO3ШмНтКѓМгЫЎжС1L |
| DЁЂ53ПЫNa2CO3ШмНтКѓМгЫЎжС0.5L |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| AЁЂЭЈШыБъзМзДПіЯТHClЦјЬх11.2LЃЌПЩЪЙ1 LХЈЖШЮЊ0.5mol/LЕФбЮЫсЮяжЪЕФСПХЈЖШдіДѓвЛБЖ |
| BЁЂ1LЫЎжаШмНтСЫ40gЧтбѕЛЏФЦЃЌИУШмвКжаNaOHЕФЮяжЪЕФСПХЈЖШЮЊ1mol/L |
| CЁЂНЋ10gЬМЫсИЦЗлФЉМгЫЎХфГЩ100mLШмвКЃЌCaCO3ЮяжЪЕФСПХЈЖШЮЊ1mol/L |
| DЁЂШєашвЊ480mL0.1mol/LЕФСђЫсЭШмвКЃЌПЩвдГЦШЁ12.5gЕЈЗЏХфГЩ500mLШмвККѓдйСПШЁЫљашЬхЛ§ШмвК |
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com