
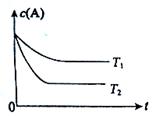
��ӦA(g) ![]() B(g) +C(g)���ݻ�Ϊ1.0L���ܱ������н��У�A�ij�ʼŨ��Ϊ0.050mol/L���¶�T1��T2��A��Ũ����ʱ���ϵ����ͼ��ʾ���ش��������⣺
B(g) +C(g)���ݻ�Ϊ1.0L���ܱ������н��У�A�ij�ʼŨ��Ϊ0.050mol/L���¶�T1��T2��A��Ũ����ʱ���ϵ����ͼ��ʾ���ش��������⣺
��1��������Ӧ���¶�T1���� T2��ƽ�ⳣ��K(T1)���� K(T2)��������ڡ�����С�ڡ����ڡ���
��2�����¶�T2ʱ��5min��Ӧ�ﵽƽ�⣬A��ת����Ϊ70%����ƽ��ʱ��ϵ�ܵ����ʵ���Ϊ���������� ���� �ڷ�Ӧ��ƽ�ⳣ��K=���������� ��
�۷�Ӧ��0~5min�����ƽ����Ӧ����v(A)=������������ ��
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣��ش��������⣺
��1����ӦA(g)+B(g)![]() C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0�����������������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0�����������������
����ȷ��������
��2����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)= CO (g)+ H2O (g)�� ��H1= +34.0 kJ/mol
����(g)= CO2 (g)+ H2(g) ��H2= ��7.0kJ/mol
�����ķ���ʽΪ ���ڸ������£���̬CO2����̬H2��Ӧ������̬CO����̬H2O���Ȼ�ѧ����ʽΪ ��
��3����ͼ��ʾ��ˮ�����Թ�����һö��������������۲죺
I���Թ���Һ����������������Ӧ�� ��
II���Թ���Һ���½������� ��ʴ��
III����Һ��Ϊˮ����Һ��Ϊ��ˮ���������� ����ס����ҡ�����Һ�и�ʴ���ٶȿ졣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ����һ�и߶��ڶ�ѧ�Σ�ģ�飩���Ի�ѧ�Ծ� ���ͣ������
��10�֣��ش��������⣺
��1����ӦA(g)+B(g) C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0�����������������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0�����������������
����ȷ��������
��2����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)=" CO" (g)+ H2O (g)�� ��H1=" +34.0" kJ/mol
����(g)= CO2 (g)+ H2(g) ��H2=" ��7.0" kJ/mol
�����ķ���ʽΪ ���ڸ������£���̬CO2����̬H2��Ӧ������̬CO����̬H2O���Ȼ�ѧ����ʽΪ ��
��3����ͼ��ʾ��ˮ�����Թ�����һö��������������۲죺
I���Թ���Һ����������������Ӧ�� ��
II���Թ���Һ���½������� ��ʴ��
III����Һ��Ϊˮ����Һ��Ϊ��ˮ���������� ����ס����ҡ�����Һ�и�ʴ���ٶȿ졣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ�߶��ڶ�ѧ�Σ�ģ�飩���Ի�ѧ�Ծ� ���ͣ������
��10�֣��ش��������⣺
��1����ӦA(g)+B(g) C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H
0�����������������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H
0�����������������
����ȷ��������

��2����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)= CO (g)+ H2O (g)�� ��H1= +34.0 kJ/mol
����(g)= CO2 (g)+ H2(g) ��H2= ��7.0 kJ/mol
�����ķ���ʽΪ ���ڸ������£���̬CO2����̬H2 ��Ӧ������̬CO����̬H2O���Ȼ�ѧ����ʽΪ ��
��3����ͼ��ʾ��ˮ�����Թ�����һö��������������۲죺

I���Թ���Һ����������������Ӧ�� ��
II���Թ���Һ���½������� ��ʴ��
III����Һ��Ϊˮ����Һ��Ϊ��ˮ���������� ����ס����ҡ�����Һ�и�ʴ���ٶȿ졣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�ϱ�У���߶�9�·�������ѧ�Ծ� ���ͣ������
��ԭ���⣩��8�֣���ӦA(g)+B(g)  C(g)+D(g)�����е������仯��ͼ��ʾ���ش��������⡣
C(g)+D(g)�����е������仯��ͼ��ʾ���ش��������⡣
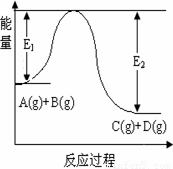
��1���÷�Ӧ��__________��Ӧ������ȡ������ȡ�����
��2������Ӧ�ﵽƽ��ʱ�������¶ȣ�A��ת����________
���������С�������䡱����
��3���ڷ�Ӧ��ϵ�м����������Ӧ��������E1��E2�ı仯
�ǣ�E1_______,E2_______���������С�������䡱����
��4����֪�����Ȼ�ѧ����ʽ��
��H2��g�� +1/2O2 ��g�� ��H2O��l������H=-285 kJ��mol-1
��H2��g�� +1/2O2 ��g�� ��H2O��g������H=-241.8 kJ��mol-1
��C��s�� +1/2O2 ��g�� ��CO��g������H=-110.5 kJ��mol-1
�� C��s�� +O2 ��g�� ��CO2��g������H=-393.5 kJ��mol-1
�ش��������⣺
�� ȼ��1gH2����Һ̬ˮ���ų�������Ϊ ��
��д��COȼ���ȵ��Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0103 ������ ���ͣ������
 C(g)+D(g)�����е������仯��ͼ��ʾ���ش��������⡣
C(g)+D(g)�����е������仯��ͼ��ʾ���ش��������⡣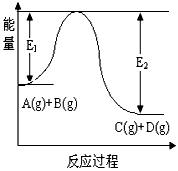
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com