
| ʵ�� | ��Ӧ�¶�/�� | Na2S2O3��Һ | ϡH2SO4 | H2O | ||
| V/mL | c/��mol•L-1�� | V/mL | c/��mol•L-1�� | V/mL | ||
| A | 25 | 5 | 0.1 | 10 | 0.1 | 5 |
| B | 25 | 5 | 0.2 | 5 | 0.2 | 10 |
| C | 35 | 5 | 0.1 | 10 | 0.1 | 5 |
| D | 35 | 5 | 0.2 | 5 | 0.2 | 10 |
���� ��1�������Ȼ�ѧ����ʽ��ע��������д��
��2�����ø�˹���ɢ�-��-�۵ã�2CO��g��+SO2��g��=CO2��g��+S��s����H=-270kJ/mol
��3��ʵ��A��ʵ��B��ʵ��B��Na2S2O3��H2SO4��
Ũ�ȶ���Ӧ���ʴ�ʵ��C��ʵ��
D���¶ȱ�ʵ��A��ʵ��B�ߣ���Ӧ
���ʴ�ʵ��C��ʵ��
D�У�ʵ��D��Na2S2O3��H2SO4��
Ũ�ȶ���Ӧ���ʴ�����ʵ��
D��Ӧ����������ȳ��ֻ��ǣ�
��4��H+��aq��+OH-��aq���TH2O��1����H=-57.3kJ/mol
1
0.25L0.10mol/L Q
�б���Ϊ1����H=0.25L0.10mol/L��Q������Q=cm��t=4.2��10-3kJ/��g•�棩��500mL��1.0g/mL����t
����t=0.68��
��� 
���� ע�⣺�к�����ϡ���ᡢ���к����� 1 molˮ�ķ�Ӧ�ȣ���1 molˮΪ�����м��㣮


 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2SiO3ˮ��Һ�׳�ˮ������������ľ�ķ���� | |
| B�� | Fe3O4�׳����죬��������ɫͿ�� | |
| C�� | Na2CO3�׳ƴ������Ϊ���첣����ԭ�� | |
| D�� | KAl��SO4��2•12H2O�׳�����������Ϊ��ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
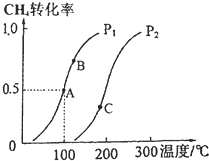 ������Ȼ�����Ƶ���H2��CO��Ϊ��Ҫ��ɵĹ�ҵԭ�Ϻϳ�������ӦΪ��CH4��g��+H2O��g��?CO��g��+3H2��g����
������Ȼ�����Ƶ���H2��CO��Ϊ��Ҫ��ɵĹ�ҵԭ�Ϻϳ�������ӦΪ��CH4��g��+H2O��g��?CO��g��+3H2��g�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | X��Y��Z��W��ԭ�Ӱ뾶���μ�С | |
| B�� | W��X�γɵĻ�������ֻ�����Ӽ� | |
| C�� | W�����������һ����Z�ĺ����������ǿ | |
| D�� | ��W��Y��ԭ���������5��������γɻ�����Ļ�ѧʽһ��ΪY2W3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | BaCl2��K2CO3��Һ��Ӧ | B�� | CO2��Ba��OH��2��Һ��Ӧ | ||
| C�� | Ba��NO3��2��Na2CO3��Һ��Ӧ | D�� | Ba��OH��2������NaHCO3��Һ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1012����Һ�У�Mg2+��Al3+��NO3-��Cl- | |
| B�� | ʹpH��ֽ����ɫ����Һ�У�NH4+��NO3-��SO42-��Na+ | |
| C�� | ��ˮ�����c��H+��=1��10-14mol•L-1����Һ�У�Mg2+��K+��Cl-��NO3- | |
| D�� | pH=0����Һ�У�K+��Fe3+��SO42-��SCN- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����̬ԭ����7��������ͬ�ĵ��ӣ�
�����̬ԭ����7��������ͬ�ĵ��ӣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����������У�±��Ԫ�أ�F��Cl��Br��I���ĵ��ʼ���������;�㷺��
�����������У�±��Ԫ�أ�F��Cl��Br��I���ĵ��ʼ���������;�㷺��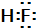 ����HF��HCl��������з����HF�ķ�����������
����HF��HCl��������з����HF�ķ�����������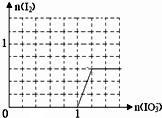 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com