��ͼ�ش��������⣨���������ѱ������ڵ��������߲����������Ը�����Ҫ���ӣ�
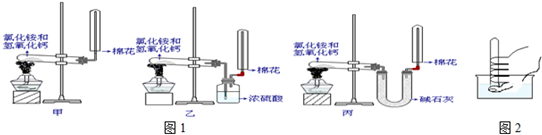
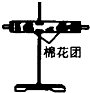
��1����������߲��������ռ�װ�ã���ͼ��װ�ÿ�����ʵ������ȡ��������ش��������⣺
�ٿ�����
ƿ�������ſ�����
ƿ�������ſ�����
�����ռ��������������ƿ�ռ���������������������μ��鰱�����ռ���
��ʪ��ĺ�ɫʯ����ֽ����ƿ�ڣ���ֽ������˵�����ռ���
��ʪ��ĺ�ɫʯ����ֽ����ƿ�ڣ���ֽ������˵�����ռ���
��
�����â����ռ��İ������������Ȫʵ�飬����Ϊ��������
��������ˮ
��������ˮ
���������ʣ�
��д��ʵ�����ư����Ļ�ѧ��Ӧ����ʽ
2NH4Cl+Ca��OH��2�TCaCl2+2NH3��+2H2O
2NH4Cl+Ca��OH��2�TCaCl2+2NH3��+2H2O
��
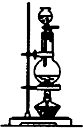
��2����ͼװ�û������������ijЩ�����������ȷֽ��ʵ�飮��ش�
��Na
2CO
3��NaHCO
3��������������һ�ֿ�������ͼװ�ý������ȷֽ��ʵ�飮���������ȷֽ�Ļ�ѧ��Ӧ����ʽΪ��
��
��Ϊ����֤���и��������ȷֽ���������������������ߴ�Ӧ���ӣ�����ʱ��ָ��ʹ��������������ʢװ���Լ�������������˳��
������װ�а�ɫ��ˮ����ͭ����ĸ���ܣ�������װ�г���ʯ��ˮ���ձ�
������װ�а�ɫ��ˮ����ͭ����ĸ���ܣ�������װ�г���ʯ��ˮ���ձ�
��
�������һ���й�ʵ������а�ȫ����Ľ��飺
��Ӧ���ȳ������ܣ��ٳ��ƾ���
��Ӧ���ȳ������ܣ��ٳ��ƾ���
��
�������12.6g�ĸ����ʼ��ȷֽ⣬����һ��ʱ����ʣ���������Ϊ9.5g�����Ѿ��ֽ�ĸ����ʵ�����Ϊ
8.4
8.4
g��

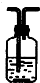
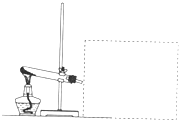
 CaCl2+2H2O+2NH3���������ͼ�������ˮ�����Բ�������ˮ���ռ��������£���������������Ӧ���Ұ����ܶ�С�ڿ��������Կ��Բ��������ſ������ռ������ռ�װ��ͼΪ��
CaCl2+2H2O+2NH3���������ͼ�������ˮ�����Բ�������ˮ���ռ��������£���������������Ӧ���Ұ����ܶ�С�ڿ��������Կ��Բ��������ſ������ռ������ռ�װ��ͼΪ��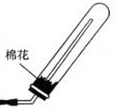 ��
�� CaCl2+2H2O+2NH3����
CaCl2+2H2O+2NH3����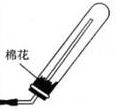 ��
��


 ��ͼ�ش��������⣨���������ѱ������ڵ��������߲����������Ը�����Ҫ���ӣ�
��ͼ�ش��������⣨���������ѱ������ڵ��������߲����������Ը�����Ҫ���ӣ� ��ͼ�ش��������⣨���������ѱ������ڵ��������߲����������Ը�����Ҫ���ӣ�
��ͼ�ش��������⣨���������ѱ������ڵ��������߲����������Ը�����Ҫ���ӣ�