
�л������һ�����ϣ���ϳ�·����ͼK294��ʾ��ϩ��A����Է�������Ϊ56����˴Ź���������ʾֻ������壻�����廯����D���Է���������Ӧ���ڴ������ڵ������£�1 mol D��2 mol H2��Ӧ���������ң����к���������CH3��

ͼK294
��֪��R��CH===CH2 R��CH2CH2OH
R��CH2CH2OH
(1)A�Ľṹ��ʽΪ________________��
(2)D�����������ŵ�������________________��D���ܷ����ķ�Ӧ������________(�����)��
a��ȡ����Ӧ b���Ӿ۷�Ӧ
c����ȥ��Ӧ d��������Ӧ
(3)�����ҷ�Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________
________________________________________________________________________��
(4)д�����һ�Ϊͬ���칹�������������������л���Ľṹ��ʽ��________________________________________________________________________��
a����FeCl3��Һ����ɫ
b�������ϵ�һ��ȡ����ֻ��2��
(1)(CH3)2C===CH2
(2)̼̼˫����ȩ����c
(3)(CH3)2CHCOOH����CH2CH2CH2OH (CH3)2CHCOOCH2CH2CH2����H2O
(CH3)2CHCOOCH2CH2CH2����H2O
(4)HO��HO
[����] (1)����ϩ��A����Է���������֪��A�ķ���ʽΪC4H8���������к�������Hԭ��ʱ����ϩ���Ľṹ��ʽΪCH3CH===CHCH3��CH2===C(CH3)2���������е���֪������֪A�Ľṹ��ʽΪ���ߡ����A�Ľṹ��ʽ����ϵ��Ŀ�еġ���֪������B�Ľṹ��ʽΪ(CH3)2CHCH2OH��CΪ��B������õ���ȩ����ṹ��ʽΪ(CH3)2CHCHO����ΪC�е�ȩ����������õ��IJ���ʼĽṹ��ʽΪ(CH3)2CHCOOH�����ݼ����ҷ�Ӧ���ɱ���������֪���÷�ӦΪ������Ӧ�������ֻ��������������Ҫ���ҷ�������������Ľṹ��ʽΪ(CH3)2CHCOOCH2CH2CH2�����ҵĽṹ��ʽΪ��CH2CH2CH2OH����D�ܷ���������Ӧ���ʷ����к���ȩ������ 1 mol D����2 mol H2�����ӳɷ�Ӧ�����ң���D�Ľṹ��ʽΪCHCHCHO��
(2)��ΪD�Ľṹ��ʽΪ��CH===CHCHO�����������Ĺ����ŵ�����Ϊ̼̼˫����ȩ���������б����ϵ�Hԭ�ӿ��Է���ȡ����Ӧ�������к���̼̼˫�����ܷ����Ӿ۷�Ӧ�����������ǻ���±ԭ�ӣ����ܷ�����ȥ��Ӧ�������е�̼̼˫����ȩ�����ܷ���������Ӧ��
(3)�����к����Ȼ����ҷ����к��д��ǻ����ʶ��߷���������Ӧ��
(4)��FeCl3��Һ����ɫ��Ҫ������к��з��ǻ��������ϵ�һ��ȡ����ֻ��2��ʱ��Ҫ��÷����к���2�����ڶ�λ��ȡ�����������������֣���CH2CH2CH3��(CH3)2CH�����ʸ�ͬ���칹��Ľṹ��ʽΪHO��HO��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ˮ������Ũ��ˮ��Դ������������ۺ����ú�ˮ����Ҫ;��֮һ��һ�����Ƚ���ˮ������õ�ˮ���ٴ�ʣ���Ũ��ˮ��ͨ��һϵ�й���������ȡ������Ʒ��
�ش��������⣺
(1)���иĽ����Ż���ˮ�ۺ����ù��յ�������������е���________(�����)��
���û�������ȡ��ˮ
����߲��ֲ�Ʒ������
���Ż���ȡ��Ʒ��Ʒ��
�ܸĽ��ء��塢þ�ȵ���ȡ����
(2)���á���������������Ũ��ˮ����Br2�����ô������ա������������Ҫ��Ӧ��Br2��Na2CO3��H2O��NaBr��NaBrO3��NaHCO3������1 mol Br2ʱ��ת�Ƶĵ�����Ϊ________mol��
(3)��ˮ��þ��һ�ι�����������ͼ��
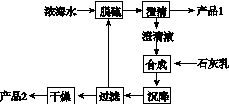
Ũ��ˮ����Ҫ�ɷ����£�
| ���� | Na�� | Mg2�� | Cl�� | SO |
| Ũ��/(g��L��1) | 63.7 | 28.8 | 144.6 | 46.4 |
�ù��չ����У��������Ҫ��Ӧ�����ӷ���ʽΪ______________________________����Ʒ2�Ļ�ѧʽΪ__________��1 LŨ��ˮ���ɵõ���Ʒ2������Ϊ________g��
(4)����ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪ________________________�����ʱ����������ˮ���ڻ���ɲ�Ʒþ�����ģ�д���йط�Ӧ�Ļ�ѧ����ʽ��____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Һ��ֻ���ܺ���K+��NO3−��SO42−��NH4+��CO32−�еļ��֣�������������H+��OH−����ȡ200 mL����Һ�ֳ����ȷݣ���������ʵ�飺һ�ݼ��������ռ���ȣ����������ڱ�״����Ϊ224 mL����һ���ȼ����������������ټ�������BaCl2�õ�2.33 g���壬�����Һ�� ��
A�����ܺ���K+ B���϶�����NO3−��SO42−��NH4+��CO32−
C��һ��������NO3− D��һ������K+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������(����)
A��SO2ʹ��ˮ��ɫ����ϩʹKMnO4��Һ��ɫ��ԭ����ͬ
B���Ʊ���������ʱ�����ȵ�NaOH��Һ�ռ������Գ�ȥ���е�����
C���ñ���ʳ��ˮ���ˮ����ʯ��Ӧ�����Լ�����Ȳ�IJ�������
D����AgNO3��Һ���Լ���KCl��KI
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�������ͬ���칹����Ŀ���ٵ���(����)
A������ B���촼 C����ϩ D����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ��������ͼ��ʾ������T������������������������ȡ������жϲ���ȷ����(����)
A�������̬�⻯������ȶ��ԣ�R>Q
B������������Ӧˮ��������ԣ�Q<W
C��ԭ�Ӱ뾶��T>Q>R
D����T������Һһ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��W�Ƕ����ڵ�Ԫ�أ�ԭ���������ε�����X��Zλ��ͬһ���壬YԪ�صĵ��ʼ��������ᷴӦҲ����NaOH��Һ��Ӧ��Zԭ�ӵ������������Ǵ�����������һ�룬Y��Z��Wԭ�ӵ�����������֮��Ϊ14������˵����ȷ����(����)
A��ԭ�Ӱ뾶��С�����˳��ΪX��Y��Z��W
B��Z���������������ˮ��Ӧ������Ӧ����
C��X��Z��W����������Ӧˮ�������Ե�ǿ��˳��ΪZ��X��W
D�������£�0.1 mol/L W����̬�⻯���ˮ��Һ��pH>1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڶ����ԭ���У������������������Щ���ؾ���
�ٵ��Ӳ��ԭ�ӹ�����ͣ������Dz㣩�ۿռ���չ���������״̬
A���٢ڡ���B���٢ܡ���C���ڢۡ���D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾ���ձ��ж�ʢ��ϡ���ᡣ
 |
��1��A�з�Ӧ�����ӷ���ʽΪ ��
��2��B�еĵ缫��Ӧ��Fe�� ��Sn�� ��Sn��������Һ��pH��������С�䣩 ��
��3��C�б���ʴ�Ľ����� ����缫��ӦʽΪ ��
�Ƚ�A��B��C�д�������ʴ�������ɿ쵽����˳���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com