
����Ŀ��ijѧ��Ϊ�ⶨij�ռ���Ʒ��![]() ��������������������ʵ�飺����֪����Ʒ�к����������������õ����ʣ�
��������������������ʵ�飺����֪����Ʒ�к����������������õ����ʣ�
A.��250![]() ������ƿ�ж��ݣ����Ƴ�250
������ƿ�ж��ݣ����Ƴ�250![]() �ռ���Һ��
�ռ���Һ��
B.�ü�ʽ�ζ�����ȡ25.00![]() �ռ���Һ����ƿ�У����μ��μ���ָʾ����
�ռ���Һ����ƿ�У����μ��μ���ָʾ����
C.����ƽ��ȷ��ȡ20.5![]() �ռ���Ʒ�����ձ���������ˮ�ܽ⣻
�ռ���Ʒ�����ձ���������ˮ�ܽ⣻
D.�����ʵ���Ũ��Ϊ1.00![]() �ı�������Һװ����ʽ�ζ����У�����Һ�棬���¿�ʼʱ�Ķ�����
�ı�������Һװ����ʽ�ζ����У�����Һ�棬���¿�ʼʱ�Ķ�����
E.����ƿ�µ�һ�Ű�ֽ���ζ�����Һ��Ϊ��ɫΪֹ�����¶�����
����գ�
(1)��ȷ���������˳����________��________��________��________��________��������ĸ��գ�
(2)�۲�ζ���Һ��Ķ���ʱӦע��ʲô���⣿
_____________________________________________________________________��
(3)![]() �������е���ƿ�µ�һ�Ű�ֽ��������__________________________________________��
�������е���ƿ�µ�һ�Ű�ֽ��������__________________________________________��
(4)���в����п���ʹ����![]() ��Һ����������ƫ�͵���________��
��Һ����������ƫ�͵���________��
a��![]() ��������δ����Һ��ȴ�����¾�ת�Ƶ�����ƿ�ж���
��������δ����Һ��ȴ�����¾�ת�Ƶ�����ƿ�ж���
b��![]() �������У�����ҩƷʱ������������̣�
�������У�����ҩƷʱ������������̣�![]() ��������
��������
c��![]() ����������ʽ�ζ�����װ���
����������ʽ�ζ�����װ���![]() ��Һǰδ�ñ�Һ��ϴ
��Һǰδ�ñ�Һ��ϴ
d���ζ������У���ȡ������Һ���ʱ����ʼʱ���Ӷ���������ʱ���Ӷ���
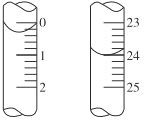
(5)����ij�������ĩ������ͼ��ʾ��δ����Ϊ________![]() ��������Ϊ________
��������Ϊ________![]() ������Ϊ________
������Ϊ________![]() �����ζ��������ݼ����ռ���Ʒ��
�����ζ��������ݼ����ռ���Ʒ��![]() ����������Ϊ________��
����������Ϊ________��
���𰸡�CABDE �����밼Һ����͵�ƽ�룻����������0.01mL ʹ�ζ��յ�ʱ����Һ��ɫ�仯�����ԣ����ڷֱ� b��d 24.00 0.30 23.70 92.49%
��������
(1)������������ҺȻ����еζ�����
(2)������ȷ�۲�ζ���Һ��Ķ���������ɣ�
(3)��ֽ����ʹ��Һ��ɫ�仯�����ԣ�
(4)a�������ȵ���Һ�����ƫ����������Ƶ�����������Һ��Ũ��Ӱ�죻b�����ݻ�ʹ����ҩƷ������С��20.5g�жϣ�c�����ݱ�Һ��ϡ�ͣ�Ũ�ȼ�С���ζ�ʱ���ĵı�Һ���ƫ���жϣ�d����ʼʱ���Ӷ��������¶���ƫ����ʱ���Ӷ��������¶���ƫС�����յ��¶���ƫС��
(5)���ݵζ��ܵĹ��켰ͼʾ�����ζ��ܵĶ������������ĵı�Һ�������Ũ�ȼ�����������Ƶ�Ũ�ȣ��ټ�����ռ���Ʒ��NaOH������������
(1)��ȷ�IJ���˳��Ϊ���������ܽ����������ζ������Բ��������˳��Ϊ��CABDE���ʴ�Ϊ��C��A��B��D��E��
(2)�۲�ζ���Һ��ʱ����Ӧ���밼Һ����͵�ƽ�룬����������0.01mL���ʴ�Ϊ�������밼Һ����͵�ƽ�룻����������0.01mL��
(3)�ζ��յ�ʱ����ֽ��������ã����ֱ���ɫ�仯���ʴ�Ϊ��ʹ�ζ��յ�ʱ����Һ��ɫ�仯�����ԣ����ڷֱ棻
(4)a��δ����Һ��ȴ�����¾�ת�Ƶ�����ƿ�ж��ݣ���ʹ����NaOH��Һ��Ũ��ƫ�Ӷ�ʹ��������ƫ�ߣ���a����b������������̣�NaOH�������̣���ʹ����ҩƷ������С��20.5g����ʹ��Ʒ����������ƫ�ͣ���b��ȷ��c����ʽ�ζ�����װ���H2SO4��Һǰδ�ñ�Һ��ϴ����ʹV(H2SO4)ƫ�Ӷ�ʹ��Ʒ����������ƫ��c����d����ʼʱ���Ӷ���������ʱ���Ӷ�������ʹV(H2SO4)ƫС���Ӷ�ʹ��Ʒ����������ƫС����d��ȷ����ѡb��d��
(5)ע�����������0.01mL���ζ�����0���̶����ϣ�ĩ����Ϊ24.00mL��������Ϊ0.30mL������Ϊ23.70mL���ʴ�Ϊ��24.00��0.30��23.70��
(6)�������Ƶ���Һ��Ũ��Ϊ��c(NaOH)=![]() =
=![]() =1.896molL-1���ռ���Ʒ��NaOH����������Ϊ��w(NaOH)=
=1.896molL-1���ռ���Ʒ��NaOH����������Ϊ��w(NaOH)=![]() ��100%=92.49%���ʴ�Ϊ��92.49%��
��100%=92.49%���ʴ�Ϊ��92.49%��


 ÿ�α���ϵ�д�
ÿ�α���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ�Ǿ����Դ���⣬�Ӻ�ˮ����ȡʳ�κ���Ĺ�����ͼ��

��1����ȥ���������ʣ�Mg2+��SO42����Ca2+���������ҩƷ˳����ȷ����______��
A��NaOH��Һ��̼������Һ���Ȼ�����Һ�����˺������
B���Ȼ�����Һ������������Һ��̼������Һ�����˺������
C��NaOH��Һ���Ȼ�����Һ��̼������Һ�����˺������
D��̼������Һ������������Һ���Ȼ�����Һ�����˺������
(2)д�����з�����Ӧ�����ӷ���ʽ��______��
(3)�������Ͽ��ǣ���������Ҳ�����յ���______
A��NaOH![]()
![]()
![]()
(4)����������Ӧ�ж�SO2��Cl2��Br2����������������ǿ������˳����______��
(5)��֪ij��Һ��Cl����Br����I�������ʵ���֮��Ϊ3��4��5������ʹ��Һ�е�Cl����Br����I�������ʵ���֮�ȱ��3��2��1����ôҪͨ�����������ʵ�����ԭ��Һ��I�������ʵ�����______
A��1/2B��1/10C��3/10D��1/5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij100mLϡ��Һ��ֻ����Fe3+��Cu2+��H+��NO3-�������ӣ��������������ۣ���Һ��Fe2+��Ũ�Ⱥͼ������۵������Ĺ�ϵ��ͼ��ʾ����������Ӧ��������Һ������������仯��������˵���в���ȷ����

A. ԭ��Һ��c(H+)=4mol��L��1
B. ��a=3����ԭ��Һ��c(Cu2+)=1mol��L��1
C. ԭ��Һ�е�c(NO3-)=7mol��L-1
D. BC�η�����Ӧ�����ӷ���ʽΪCu2++Fe=Fe2++Cu
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飺
(1)��һ�������л���A�����������г��ȼ��,ʵ���ã�����5.4gH2O��8.8gCO2����������6.72L(��״����)��������ʵ�ʵ��ʽ��____________��
(2)�������Dzⶨ���л����������Է����������õ���ͼ1��ʾ������ͼ��������Է�������Ϊ_____________�������ʵķ���ʽ��_____________��
(3)��Ԥ��A�Ŀ��ܽṹ��д���ṹ��ʽ��_____________��
(4)�˴Ź��������ܶ��л�������в�ͬλ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ(�ź�)�����ݷ�ֵ(�ź�)����ȷ����������ԭ�ӵ��������Ŀ��������ȼ���(ClCH2OCH3����2����ԭ��)�ĺ˴Ź���������ͼ2��ʾ��
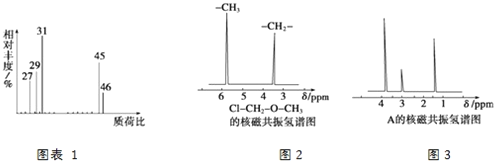
���ⶨ���л���A�ĺ˴Ź�������ͼ��ͼ3��ʾ����A�Ľṹ��ʽΪ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I��������7��18�ĸ��ӣ���Ҫ������

��1���÷�յ�ʵ���Ԫ�����ڱ���������_______________
��2������ը������ָ����200������ǰ���������������ʶ�������һ���ܶ������¶����ߵ�ԭʼ���У�����ij��ԭ���������˱�ը�����Ȳ��������ӡ����Ӻ͵��ӣ����Ͳ�����Ԫ�أ���֪����Ԫ�ص�һ�ֺ���û�����ӡ����ɴ˿�֪���Ȳ�����Ԫ����________ (��дԪ������)����Ԫ�ص���һ�ֺ��أ�������������������1�����ֺ��ص�ԭ�ӷ�����________��
��3��C��D��E��F�ļ����Ӱ뾶��С�����˳����____________(��д���ӷ���)��
��4�����õ���ʽ��ʾ��E��H��ɵĻ�������γɹ��̣�_________________��
��5����ʵ���������ǽ����ķֽ��ߣ���ʵ�������Ԫ�صķֽ��ߡ�_____________
��6����д��B�γɵĵ�����������Ӧ�Ļ�ѧ����ʽ��______________��
��7�����и��������У���ȷ����__________��
a.��ѧ���ɷ�Ϊ���Ӽ������ۼ������Լ��ͷǼ��Լ���������
b.�ɷǽ���Ԫ����ɵĻ����ﲻһ���ǹ��ۻ�����
c.�Ǽ��Լ�ֻ������˫ԭ�ӵ��ʷ�����
d.��Na2S��Һ�м�����ˮ����Һ�г��ֻ�ɫ����������˵����Ԫ�صķǽ����Ա���Ԫ��ǿ
e.±�ط����ȡ��塢����⻯��ķе��ɵ͵��ߵ�˳��Ϊ��HF<HCl<HBr<HI
f.���ɵ��ʷ��ӵ����Ӳ�һ�����й��ۼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������˵����ȷ����(����)
A. ��״���£�0.1 mol Cl2����ˮ��ת�Ƶĵ�����ĿΪ0.1NA
B. 25 �棬pH��13��NaOH��Һ�к���OH������ĿΪ0.1NA
C. ��״���£�4.48 L SO3�����ķ�����ĿΪ0.2NA
D. ���³�ѹ�£�16gO2��O3������庬�е���ԭ����ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȼ��0.1 molij�л����0.2 molC02��0.3 molH20���ɴ˵ó��Ľ��۲���ȷ����
A. ���л�������п��ܺ�����ԭ��
B. ���л�����̼����Ԫ��ԭ����Ŀ֮��Ϊ1:3
C. ���л�������в����ܺ���![]() ˫��
˫��
D. ���л�����ӵĽṹ��ʽΪCH3-CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
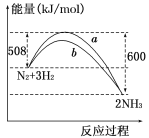
����Ŀ��(1)��ͼ��ij�¶��£�N2��H2��Ӧ�����������仯������ͼ���÷�Ӧ���Ȼ�ѧ����ʽΪ___________________________________________________________��
(2)��֪���з�Ӧ�ķ�Ӧ�ȣ�
��CH3COOH(l)+2O2(g)=2CO2(g)+2H2O(l) ��H1=��870.3kJ/mol
��C(s)+O2(g)=CO2(g) ��H2=��393.5kJ/mol
��H2(g)+1/2O2(g)��H2O(l) ��H3=��285.8kJ/mol
�Լ���������Ӧ�ķ�Ӧ��2C(s)+O2(g)+2 H2(g)��CH3COOH(l)_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪![]() ��������10mol/L����Һ����ȣ����������������к�ɫ���������������̼�����ζ����������Һ������ɫ���ݴ��ж�����˵����ȷ����
��������10mol/L����Һ����ȣ����������������к�ɫ���������������̼�����ζ����������Һ������ɫ���ݴ��ж�����˵����ȷ����
A. ��Ӧ��������������
B. ![]() ����Ԫ�ر�����
����Ԫ�ر�����
C. �̼�����ζ�������ǰ���
D. 1mol![]() ��ȫ��Ӧת��0.5mol����
��ȫ��Ӧת��0.5mol����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com