
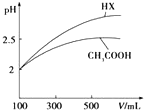
 ��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��
��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ����ƽ�ⳣ�� | Ka=1.8��10-5 | Kal=4.3��10-7 | Ka2=5.6��10-11 | Ka=3.0��10-8 |
���� ��1������ĵ���ƽ�ⳣ��Խ��������Խǿ���������ˮ��̶�Խ����
��2��0.1mol/L��CH3COOH��Һ��ˮϡ�����У��������������������ʵ���������������ʵ�����С��Ũ�ȼ�С�����Լ�����ˮ�����ӻ��������䣬����ĵ���ƽ�ⳣ�����䣻
��3����ͼ��������ˮϡ�͵Ĺ����У�HX��pH�仯�ȽϿ죬˵��HX�����Աȴ���ǿ��
��4��������Һ�еĵ���غ�������غ������㣻
��5��n��CO2��=$\frac{1.12L}{22.4L/mol}$=0.05mol��n��NaOH��=0.75mol/L��0.1L=0.075mol��1��$\frac{n��NaOH��}{n��C{O}_{2}��}$��2��
��Һ�д���̼���ƺ�̼�����ƣ���̼���Ƶ����ʵ���Ϊx��̼�����Ƶ����ʵ���Ϊy�������������غ㡢̼ԭ���غ��$\left\{\begin{array}{l}{2x+y=0.075}\\{x+y=0.05}\end{array}\right.$���$\left\{\begin{array}{l}{x=0.025}\\{y=0.025}\end{array}\right.$����Һ��Һ�е�����Ϊ�����ʵ�����̼���ƺ�̼�����ƣ���ϵ���غ�������غ��жϣ�
��� �⣺��1���ݵ���ƽ�ⳣ����֪��������ǿ������˳��Ϊ��CH3COOH��H2CO3��HClO��HCO3-�����������Խ����������ӵ�ˮ��̶�Խ����Һ����Խǿ������pH��С��������˳����a��d��c��b���ʴ�Ϊ��a��d��c��b��
��2��0.1mol/L��CH3COOH��Һ��ˮϡ�����У��������������������ʵ�������Ũ�ȼ�С�����Լ�����A��������Ũ�ȼ�С���ʴ���
B����ˮϡ�����У����������ʵ���������������ʵ�����С������$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$������ȷ��
C��ˮ�����ӻ��������䣬�ʴ���
D��������Һ��ˮϡ��ʱ���Լ�����������Ũ�ȼ�С����������Ũ����������$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$������ȷ��
E������ĵ���ƽ�ⳣ�����䣬�ʴ���
�ʴ�Ϊ��BD��
��3����ͼ��������ˮϡ�͵Ĺ����У�HX��pH�仯�ȽϿ죬˵��HX�����Աȴ���ǿ��HX�ĵ���ƽ�ⳣ���ȴ���ʴ�Ϊ�����ڣ�ϡ����ͬ������һԪ��HX��pH�仯��CH3COOH�Ĵ�HX���Խ�ǿ������ƽ�ⳣ���ϴ�
��4��CH3COOH��CH3COONa�Ļ����Һ�У����ڵ���غ㣺c��Na+��+c��H+��=c��OH-��+c��CH3COO-��������c��CH3COO-��-c��Na+��=c��H+��-c��OH-��=10-6mol/L-10-8mol/L=9.9��10-7mol/L��
�ʴ�Ϊ��9.9��10-7��
��5��n��CO2��=$\frac{1.12L}{22.4L/mol}$=0.05mol��n��NaOH��=0.75mol/L��0.1L=0.075mol��1��$\frac{n��NaOH��}{n��C{O}_{2}��}$��2��
��Һ�д���̼���ƺ�̼�����ƣ���̼���Ƶ����ʵ���Ϊx��̼�����Ƶ����ʵ���Ϊy�������������غ㡢̼ԭ���غ��$\left\{\begin{array}{l}{2x+y=0.075}\\{x+y=0.05}\end{array}\right.$�����$\left\{\begin{array}{l}{x=0.025}\\{y=0.025}\end{array}\right.$����Һ��Һ�е�����Ϊ�����ʵ�����̼���ƺ�̼�����ƣ������Ӳ�ˮ�⣬̼�������ˮ��̶ȴ���̼��������ӣ���Һ�ʼ��ԣ���������Ũ�ȴ�С˳���ǣ�
c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
���� ���⿼����������ʵĵ��룬��ȷ������ʵĵ����ص㡢����ƽ�ⳣ�����������ˮ��̶ȵĹ�ϵ�ٽ���غ�˼�������𣬣�5����Ҫ���ж���Һ�е����ʣ��ٽ������ˮ��̶���Դ�С�ж�����Ũ�ȣ��Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ĵ�Ӿ | B�� | ѪҺ��������ԭ��Ӧ | ||
| C�� | ѪҺ�з������ֽⷴӦ | D�� | ����ľ۳� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Cu��OH��2����Һ | B�� | ��ˮ | C�� | ����KMnO4��Һ | D�� | NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʳ�׳�ȥůƿ�ڵ�ˮ�� | |
| B�� | ��������Һ�м�������隣�����Һ��������ɫ���� | |
| C�� | ������Һ�мӵ�ˮ���� | |
| D�� | ��֯Ʒ����ë֯Ʒ���ֱ���������ζ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ж�һ��Ԫ���ǽ������Ƿǽ��� | B�� | �жϻ�������Ԫ�صĻ��ϼ� | ||
| C�� | �жϻ�ѧ������ | D�� | �жϻ�������ܽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ˮ���𱽡����Ȼ�̼������ϩ | |
| B�� | ��ȥ�����������ӣ��ȼ�Ũ��ˮ���ٹ��� | |
| C�� | �����Ը��������Һϴ�������Գ�ȥ������������ϩ | |
| D�� | ��ȥ���������е�������Ҵ���������������Һϴ�ӣ��ٷ�Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
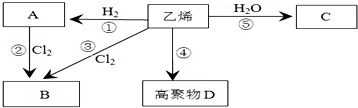
 �������к��еĹ��ۼ������зǼ��Լ������Լ�����Լ���Ǽ��Լ����� C�к������������ǻ���
�������к��еĹ��ۼ������зǼ��Լ������Լ�����Լ���Ǽ��Լ����� C�к������������ǻ���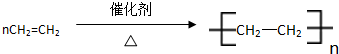 ����Ӧ���ͣ��Ӿ۷�Ӧ����CH2=CH2+H2O$\stackrel{һ������}{��}$CH3CH2OH����Ӧ���ͣ��ӳɷ�Ӧ��
����Ӧ���ͣ��Ӿ۷�Ӧ����CH2=CH2+H2O$\stackrel{һ������}{��}$CH3CH2OH����Ӧ���ͣ��ӳɷ�Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��һ�δ�ĥ����þ������������ˮ�� | |
| B�� | �� Cl2ͨ��FeCl2��Һ�� | |
| C�� | ���̶���ļ�Ͷ��ˮ�� | |
| D�� | ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 X��Y��ZΪ���������ֵ��ʣ�Z����ɫֲ�������õIJ���֮һ��A��BΪ���������������һ�������¿ɷ�����ͼ��ʾ�ķ�Ӧ�������ڷ���Һ�н��еķ�Ӧ����
X��Y��ZΪ���������ֵ��ʣ�Z����ɫֲ�������õIJ���֮һ��A��BΪ���������������һ�������¿ɷ�����ͼ��ʾ�ķ�Ӧ�������ڷ���Һ�н��еķ�Ӧ���� ��
��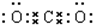 ��
��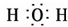 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com